According to the VSEPR theory, what is the molecular shape of #CO_2#?
1 Answer
May 5, 2016
The molecular shape of
Explanation:
The lewis structure of
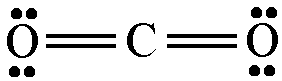
The hybridization of the central carbon atom is

