A circular medallion is to be electroplated with gold by using an electrolyte of #sf(Au_((aq))^(3+))#. Given the data below, how long will it take to plate a layer of gold #sf(1mum)# thick?
Diameter = 4 cm. Thickness = 2 mm. Density of gold = #sf(19.3"g/cm"^3)# .
#sf(F=9.6485color(white)(x)"C/""mol")# .
Current = 89 A.
Diameter = 4 cm. Thickness = 2 mm. Density of gold =
Current = 89 A.
1 Answer
Explanation:
Firstly I will find the volume of the plated medallion. Then subtract the volume of the unplated disc.
This will give me the total volume of gold. Given the density I can then get the mass of gold.
Since we know it is
Since current is the rate of flow of charge I can get the time taken to discharge this amount.
The first thing to do is put the lengths into a consistent unit as three different ones are used in the question. Since density is in
Imagine a cross section like this:
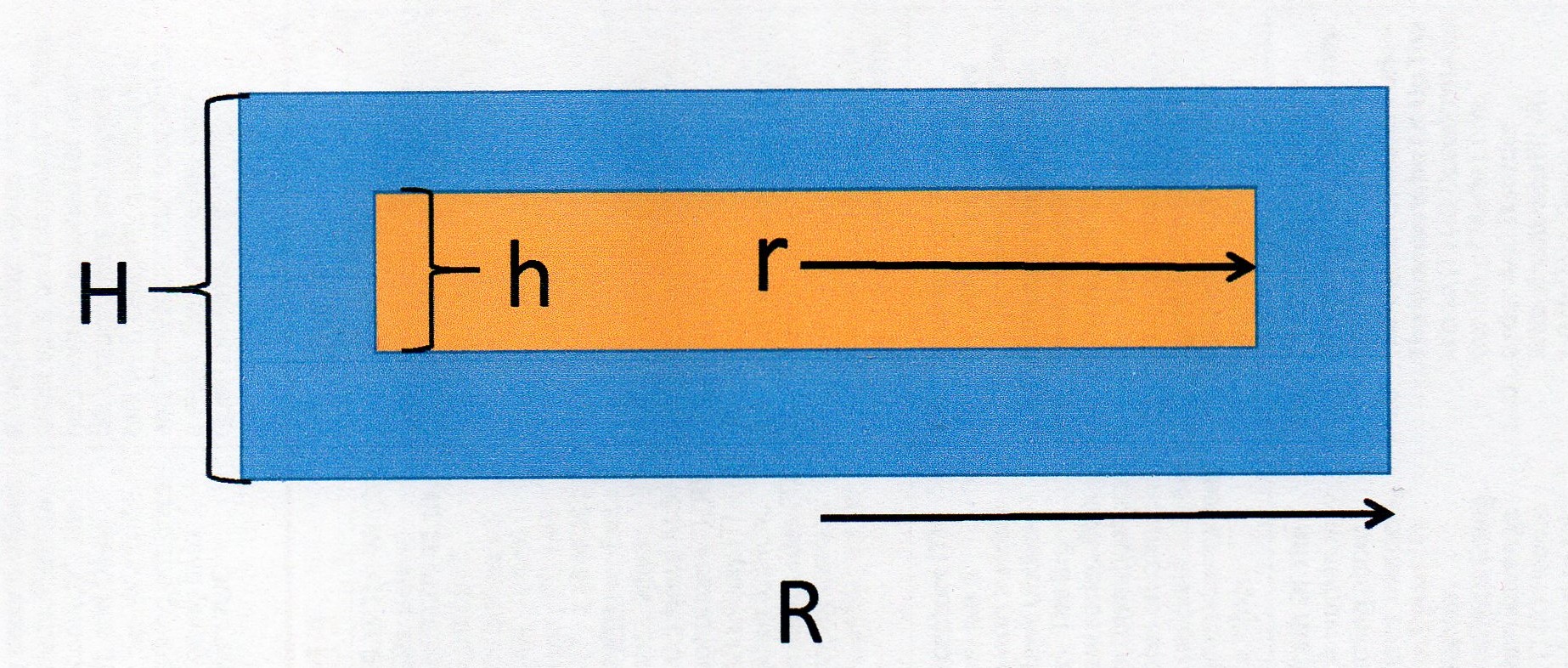
The blue area is the gold. The orange area is the medallion.
The volume of the plated disc
The thickness of the layer is
So
The medallion is
So the volume of the plated disc becomes:
The volume of the medallion
To get the volume of gold in blue we subtract
The density
The electrode equation is:
This tells us that 1 mole of
The charge on 3 moles of electrons =
The
Electric current
In reality a current of
Otherwise you get a spongy deposit which falls away easily.

