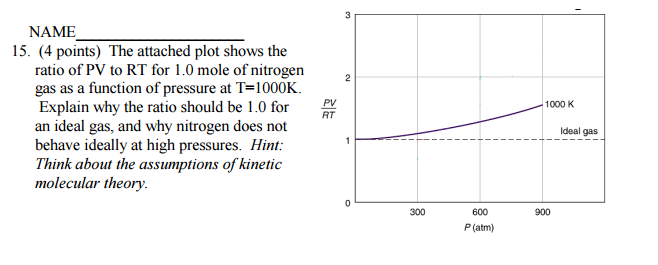
The attached plot shows the ratio of PV to RT for 1.0 mole of nitrogen gas as a function of pressure at T=1000K. Explain why the ratio should be 1.0 for an ideal gas, and why nitrogen does not behave ideally at high pressures?
1 Answer
The ideal gas law holds that
Explanation:
The ideal gas law is an idealization. What does this mean? It means that we idealize the behaviour of real gases. We assume that gases are mostly empty space, that the volume of the gaseous particles are negligible compared to the volume occupied by the gas, and that pressure is the result of the gaseous particles hitting the sides of the container elastically.
Under conditions of moderate pressure (and moderate to high temperature), all gases behave ideally. And in fact the graph shows that

