How does frequency of light affect electrons?
1 Answer
Jun 3, 2016
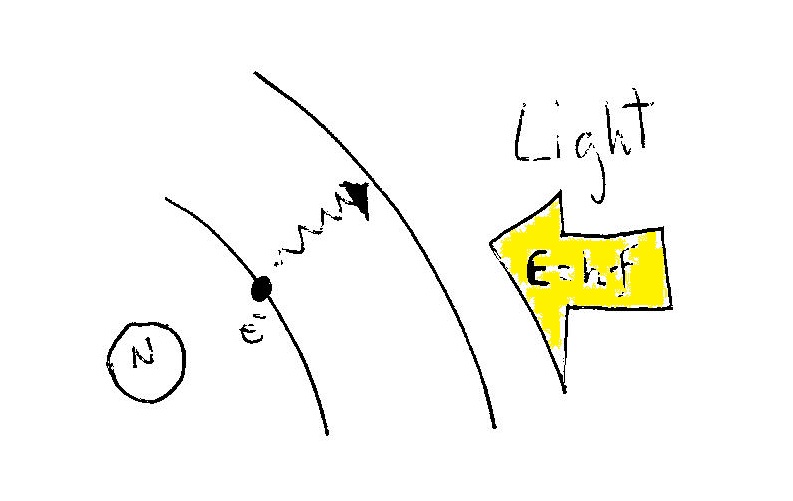
They can use the energy of light, which depends upon its frequency, to move between allowed orbits in the atom.
Explanation:
The energy
where
Electrons can absorb the right amount of energy from photons of light (of the right frequency) and jump to different allowed orbits inside the atom (as proposed by Bohr in this model of the hydrogen atom).