What is an oxidative addition reaction?
1 Answer
DISCLAIMER: You will NOT see this in first-semester inorganic chemistry.
Oxidative addition is typically an organometallic chemistry reaction wherein a molecule adds across a square-planar transition metal complex to form an octahedral complex. You should see this in an advanced inorganic chemistry class.
It tends to be paired with reductive elimination, the reverse reaction if and only if the added ligands were cis (otherwise, it is a separate reaction that, though being the reverse reaction in principle, is a different reaction). You can see more info on that here.
Conclusions we have made based on the information below:
- The oxidation state of the central metal ion increases by
#2# . - The coordination number of the metal increases by
#2# . - It can be either cis or trans addition, depending on the reaction mechanism. We don't necessarily know ahead of time.
- All
#\mathbf(16)# -electron complexes would become#\mathbf(18)# -electron complexes as a result. This is commonly seen if this occurs in a catalytic cycle.
OXIDATIVE ADDITION: CONTEXT/SETUP
A nice example of a square planar complex is Vaska's Complex
(trans
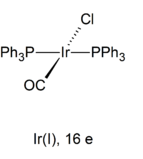
Iridium has big
Thus, trans
OXIDATIVE ADDITION: REACTION EXAMPLES
When an oxidative addition reaction occurs, the oxidation state of the central metal ion increases by
Here are some examples:
Sometimes, the product is added cis, and other times it is added trans. How it's added, however, depends on the mechanism, which is not demonstrated in my book.
What we can say about these reactions is...
REACTION 1:
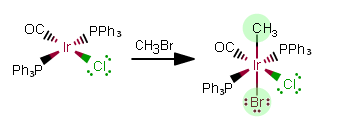
The added ligands contribute a charge to the complex, changing the oxidation state of
As free ligands in this still-NEUTRAL complex, the added ligands are
As free ligands,
That makes this an
REACTION 2:
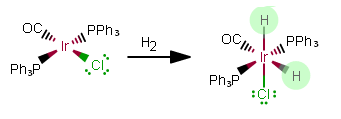
As free ligands in this still-NEUTRAL complex, the added ligands are
As free ligands,
That makes this an
REACTION 3:
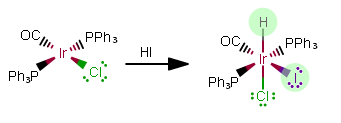
As free ligands in this still-NEUTRAL complex, the added ligands are
As free ligands,
That makes this an
WHERE MIGHT WE SEE THIS?
You'd see oxidative addition often in catalytic cycles.
These cycles are taken advantage of in industrial processes to efficiently generate materials like acetic acid (Monsanto and Cativa processes), ammonia (Haber process), sulfuric acid (Contact process), and many other useful substances we use today.
If you want to read about catalytic cycles, I've gone over the Monsanto process here.

