What is carbocation intermediate?
1 Answer
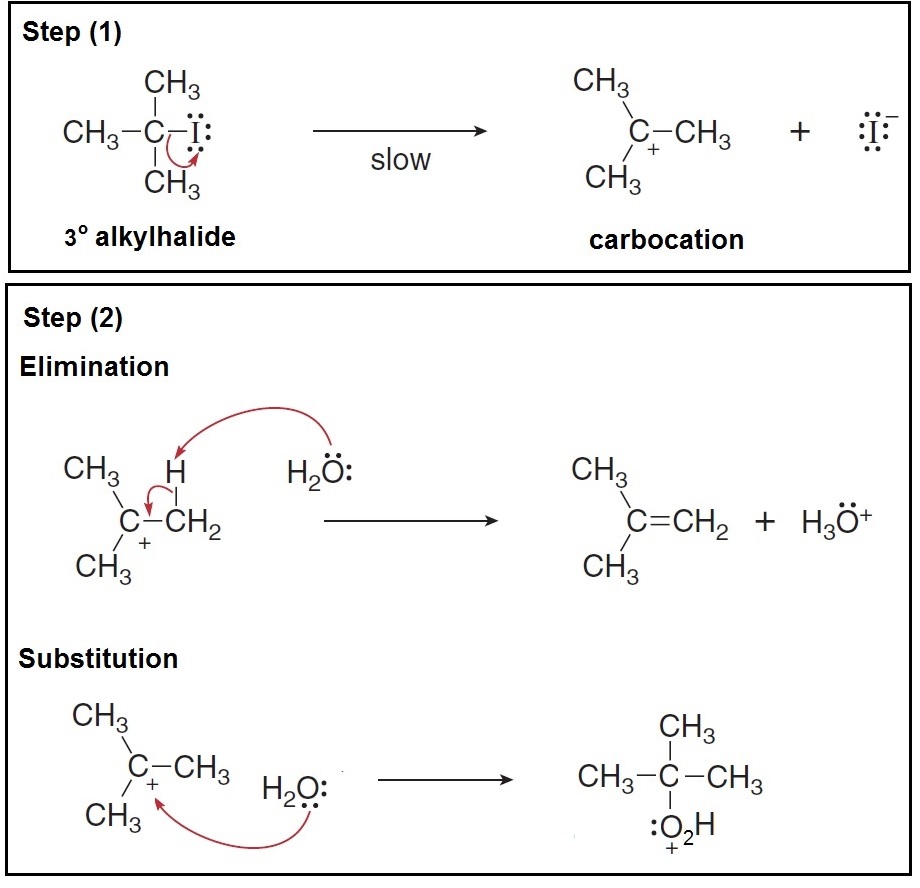
A carbocation is an organic molecule, an intermediate, that has a carbon atom bearing a positive charge and three bonds instead of four.
Explanation:
A carbocation is an organic molecule, an intermediate, that has a carbon atom bearing a positive charge and three bonds instead of four. Since the charged carbon atom does not satisfy the octet rule, it is unstable and therefore highly reactive.
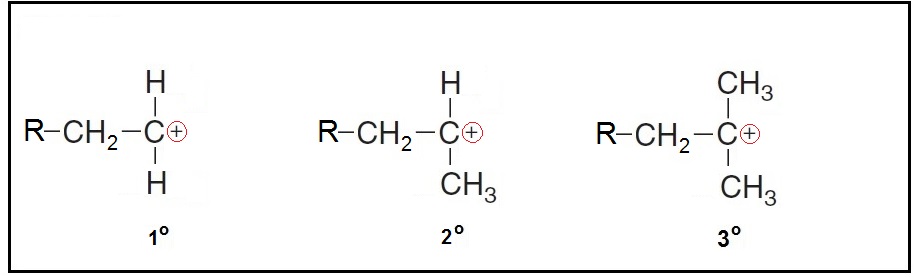
Carbonate ions are classified into primary
-
A primary carbocation has one carbon group attached to the carbon bearing the positive charge.
-
A secondary carbocation has two carbons attached to the carbon bearing the positive charge.
-
A Tertiary carbocation has three carbons attached to the carbon bearing the positive charge.)
The carbocation intermediate is a common intermediate in
The following example illustrates how a carbocation is formed when a tertiary alkyl halide is used... the carbocation could either undergo elimination or substitution...