A certain shade of blue has a frequency of 7.32 * 10^147.32⋅1014 HzHz. What is the energy of exactly one photon of this light?
1 Answer
Jun 21, 2016
Explanation:
I believe that you have to use the following equation to answer this question:
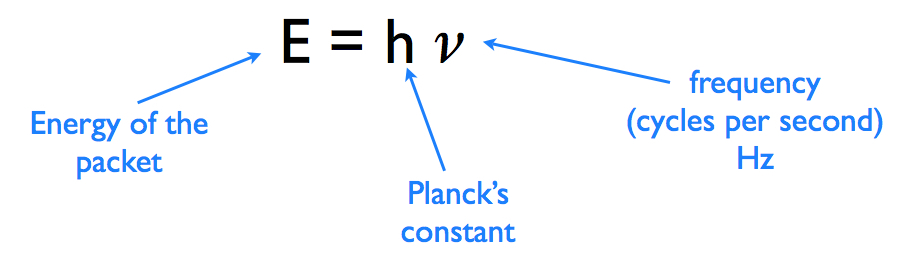 grigerscience.weebly.com
grigerscience.weebly.com
I should also note that Planck's constant has a value of
We know the frequency and Planck's constant so all we have to do is plug the given values into the equation like so:

