How do you write the radioactive decay equation for the radioisotopes, Gold-198 and Radon-222?
1 Answer
Jun 23, 2016
Hope this is the answer you were looking for.
Explanation:
Gold-198 (Au-198) is a
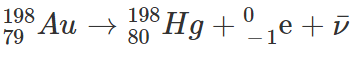
So Gold-198 (Au) decays to Mercury-198 (Hg) emitting a
Radon-222 is an alpha emitter: two protons and two neutrons are emitted from the nucleus; the equation is as follows:
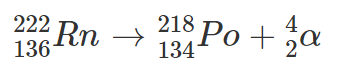
So Radon-222 (Rn) decays to Polonium-218 (Po) emitting an

