Which set(s) of of quantum numbers describe a 4d orbital?
1 Answer
A total of
Explanation:
As you know, we use four quantum numbers to describe the position and spin of an electron in an atom.
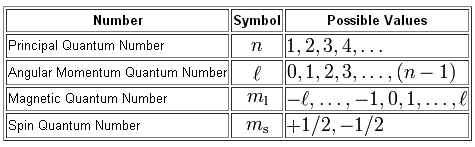
Each electron has its unique set of quantum numbers, which means that two electrons can share one, two, or even three quantum numbers, but never all four.
Now, you are given a
So, the principal quantum number,
#n = color(red)(4) -># the electron is located on the fourth energy level
The subshell in which the electron is located is described by the angular magnetic quantum number,
#l=0 -># the s-subshell#l=1 -># the p-subshell#l=2 -># the d-subshell#l=3 -># the f-subshell
Since you're looking for the d-subshell, you will need
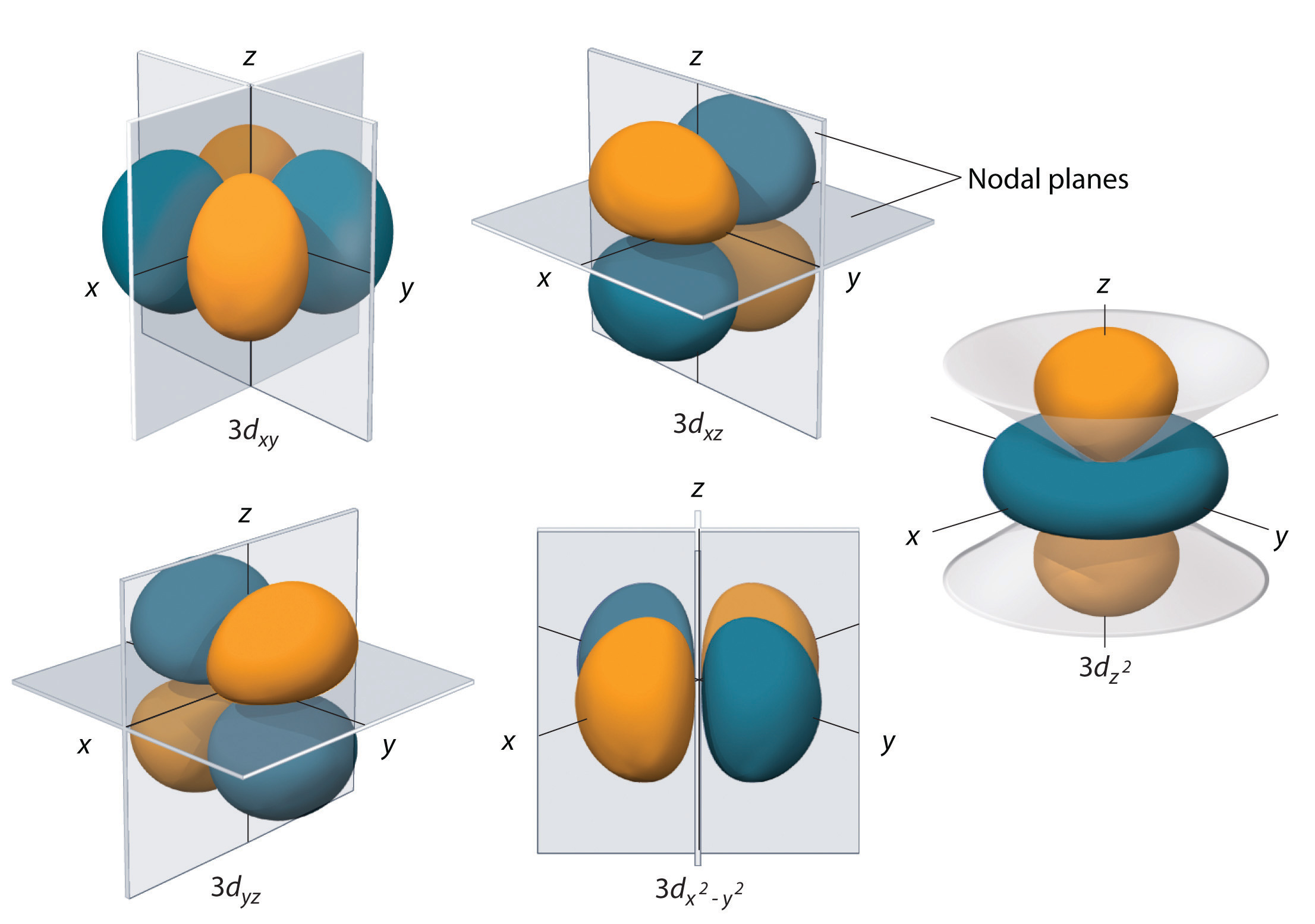
The specific orbital in which the electron is located is given by the magnetic quantum number,
#m_l = {-2, -1, color(white)(-)0, +1, +2}#
Each of these five values describes one of the five d-orbitals available in a d-subshell.
Finally ,the spin quantum number,
Now, since each orbital can hold a maximum of two electrons, one with spin-up and one with spin-down, it follows that the d-obitals can hold a total of
#"2 e"^(-)"/ orbital" xx "5 orbitals" = "10 e"^(-)#
Each of these ten electrons will have its unique set of four quantum numbers.
- all the ten electrons will share the principal and angular momentum quantum numbers
#n= color(red)(4)" "# and#" "l=2#
- five electrons will share the spin quantum number
#m_s = -1/2" "# or#" "m_s = +1/2#
- two electrons will share the magnetic quantum number
#m_l = -2" "# or#" "m_l = -1" "# or#" "m_l = color(white)(-)0" "# or#" "m_l = +1" "# or#" "m_l = +2#
You will thus have
#n=color(red)(4), l=2, m_l = -2, m_s = +1/2#
#n=color(red)(4), l=2, m_l = -2, m_s = -1/2#
#n=color(red)(4), l=2, m_l = -1, m_s = +1/2#
#n=color(red)(4), l=2, m_l = -1, m_s = -1/2#
#n=color(red)(4), l=2, m_l = color(white)(-)0, m_s = +1/2#
#n=color(red)(4), l=2, m_l = color(white)(-)0, m_s = -1/2#
#n=color(red)(4), l=2, m_l = +1, m_s = +1/2#
#n=color(red)(4), l=2, m_l = +1, m_s = -1/2#
#n=color(red)(4), l=2, m_l = +2, m_s = +1/2#
#n=color(red)(4), l=2, m_l = +2, m_s = -1/2#

