Why is it that d-block metals occupy the #d# orbitals a lot of the time, whereas f-block metals occupy the #d# orbitals much less often? Can electronic transitions occur within the valence space of f-block elements?
1 Answer
Let's see if I'm interpreting your question correctly here...
I think you're asking why:
- electrons that came from the
#4f# orbital for f-block elements are sometimes not that unstable in the#5d# orbitals. - electrons might sometimes be more stable in the
#3d# orbitals instead of the#4s# orbital for the first-period transition metals, for example. Note that however, electron transitions cannot occur straight from the#4s# to the#3d# !
It's hard to say exactly why, but here's my best guess.
4F AND 5D ORBITAL RELATIONSHIPS
Using the lanthanides as an example, the normal (non-anomalous) electron configurations include:
#"Pr" (Z = 59)# ,#[Xe] 6s^2 4f^3# - . . .
#"Eu" (Z = 63)# ,#[Xe] 6s^2 4f^7# #"Tb" (Z = 65)# ,#[Xe] 6s^2 4f^9# - . . .
#"Yb" (Z = 70)# ,#[Xe] 6s^2 4f^14#
So, our initial thought is that an excited
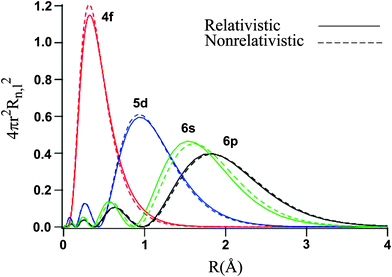
It might help to look at the radial density distribution graph, which shows the electron density map of an orbital (the likelihood of an electron appearing somewhere in an orbital, plotted over time):
You can see that the functions for the
That means that sometimes, the
In other words,
However, for the heavier elements...
1. The larger size of the
#5d# orbitals (you can see that its graph reaches out to higher radius#r# than for the#4f# ) become a significant factor to having a more stable configuration in terms of having more space.The
#5d# orbitals are larger by about#\mathbf(1)# #\mathbf(Å)# (about#"100 pm"# , the average radius of an atom).2. the
#5d# orbitals are more diffuse (their electron density is more spread out, since their graph spans mostly#0~2# #Å# , whereas that of the#4f# orbital spans mostly#0~1# #Å# ). So, on average the electrons are further apart.
It means there is, on average, less electron-electron repulsion (a destabilizing factor) when the electrons are in the
I'd say that those factors are usually the primary causes for the extra stability in the
For the lighter transition metals, we don't see as much of an influence of orbital size on "anomalies" in electron behavior, since