Question #05cff
1 Answer
Here's what I got.
Explanation:
Let's start with the oxidation numbers of nitrogen and sulfur in the sulfamate anion,
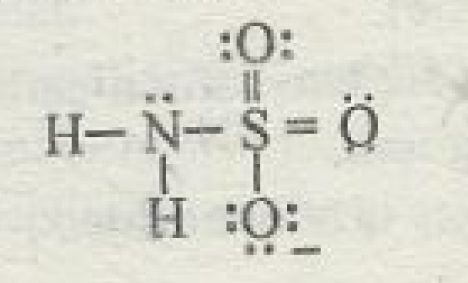
Oxidation numbers are assigned to bonded atoms based on the idea that the more electronegative of the two atoms "takes" all the bonding electrons.
Take a single bond that exists here between nitrogen and hydrogen. A single bond consists of two shared electrons, one coming from nitrogen and one from hydrogen.
Since nitrogen is more electronegative than hydrogen, it will "take" both of these bonding electrons. One already belonged to nitrogen, so that will not affect its oxidation state.
However, the second one will. Since nitrogen now has one extra electron that it "took" from hydrogen, its oxidation state will be
The exact same thing happens for nitrogen's second single bond to a hydrogen atom. It takes both bonding electrons, which means that the extra electron coming from hydrogen pushes its oxidation state to
Now, nitrogen is also more electronegative than sulfur. Since it forms a single bond with sulfur, nitrogen will once again "take" both bonding electrons.
The oxidation state of nitrogen will thus be
- one electron from each of the two hydrogen atoms
- one electron from the sulfur atom
Now look at sulfur. It already "lost" one electron to nitrogen so its oxidation state would be
Sulfur also shares two double bonds with oxygen atoms, which are more electronegative than sulfur. These atoms will each "take" the
Finally, sulfur also forms a single bond with an oxygen atom, so it will "lose" one electron to this oxygen atom.
Its oxidation state will thus be
- one electron to the nitrogen atom
- two electrons to each of the two oxygen atoms to which it forms double bonds
- one electron to the oxygen atom to which it forms a single bond
Notice that the sum of the oxidation states of each atom is equal to the overall charge of the anion
#stackrel(color(blue)(-3))("N")stackrel(color(blue)(+1))("H")_ 2 stackrel(color(blue)(+6))("S") stackrel(color(blue)(-2))("O")"" _ 3^(-)#
#1 xx (color(blue)(-3)) + 2 xx (color(blue)(+1)) + 1 xx (color(blue)(+6)) + 3 xx (color(blue)(-2)) = -1" "color(green)(sqrt())#

