How is carbocation formed?
1 Answer
Carbocations form by one of two basic mechanisms:
- Heterolytic bond cleavage by the loss of a leaving group.
- Addition of π electrons to an electrophile.
Explanation:
A carbocation is an organic molecule, an intermediate, that forms as a result of the loss of two valence electrons, normally shared electrons, from a carbon atom that already has four bonds. This leads to the formation of a carbon atom bearing a positive charge and three bonds instead of four. The whole molecule holding the positively charged carbon atom is referred to as a carbocation intermediate.
Carbocations form by one of only two basic mechanisms:
- Heterolytic bond cleavage by the loss of a leaving group. This results in the ionization of a carbon atom attached to it.
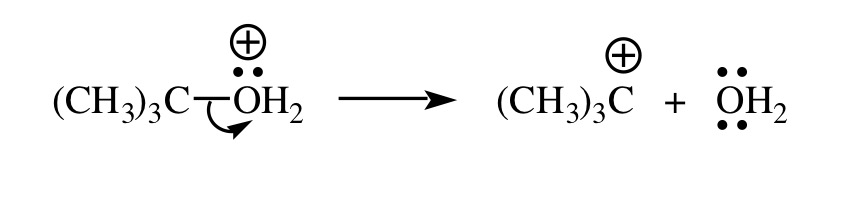
- Addition of π electrons of a π bond to an electrophile. One of the two carbon atoms involved in the π bond will have three bonds instead of four and bears the positive charge.
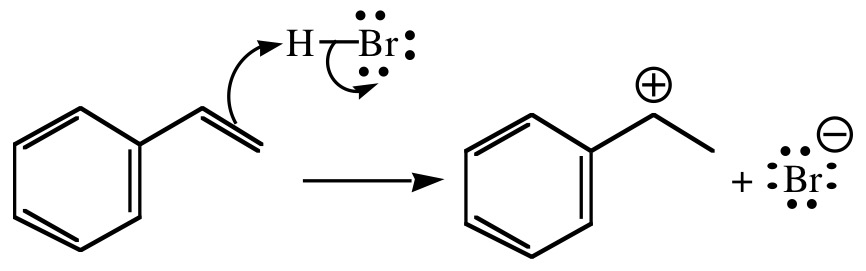
Note that the positively charged carbon atom is
