Question #09c15
1 Answer
Here's what's going on.
Explanation:
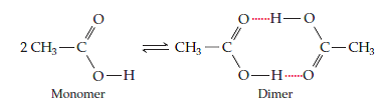
Acetic acid,
Now, the thing to remember about this equilibrium is that it lies to the left, meaning that the monomer is the dominant form, when acetic acid is in aqueous solution.
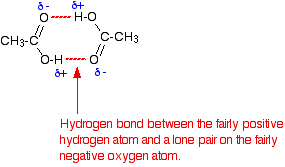
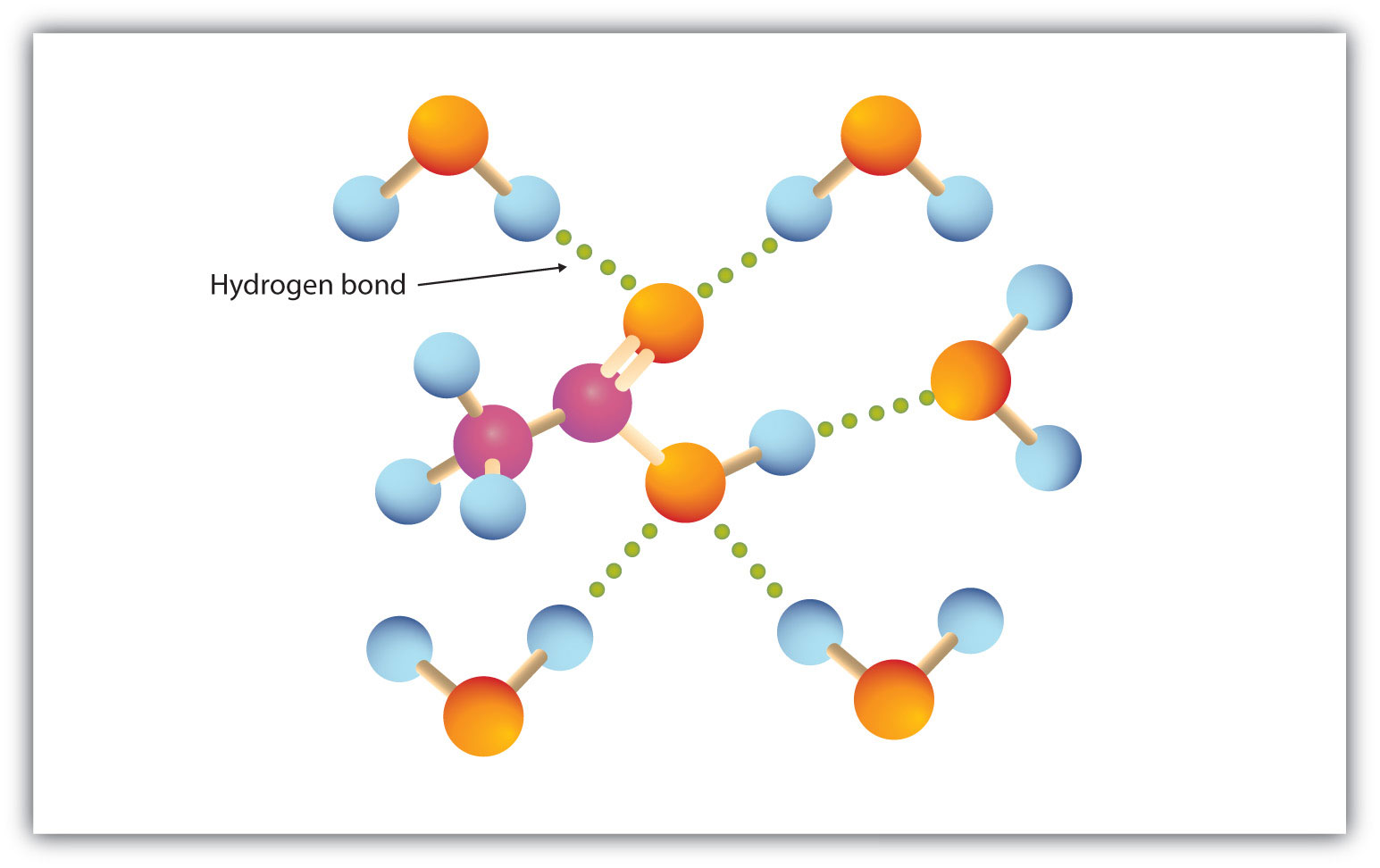
As you know, water is a polar molecule that can form hydrogen bonds because of its partial negative oxygen atom and its partial positive hydrogen atoms.
Water can thus form hydrogen bonds with a molecule of acetic acid and prevent it from forming hydrogen bonds with a similar molecule of acetic acid, i.e. acetic acid can't form dimers in aqueous solution.
On the other hand, benzene,
When acetic acid is placed in benzene, the monomer / dimer equilibrium lies to the right because there are no more polar molecules present that can disrupt the hydrogen bonding between two acetic acid molecules.
This means that the acetic acid molecules are free to form hydrogen bonds between themselves, i.e acetic acid forms dimers in benzene.
This of course implies that the molecular mass of the molecule doubles because the dimer, which is itself a chemical species, contains
#M_("M dimer") = 2 xx M_("M acetic acid")#
This gets you
#M_("M dimer") = 2 xx "60 g mol"^(-1) = "120 g mol"^(-1)#