A 4.0 L sample of hydrogen gas at 700 mmHg would occupy what volume at 250 mmHg?
1 Answer
Explanation:
We can calculate the answer using Boyle's Law which shows that there is an inverse relationship between pressure and volume as long as the temperature and number of moles remain constant.
The equation we use is:
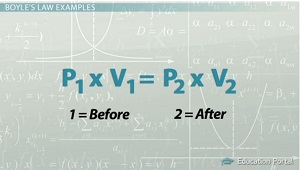
The first volume has a value of
All we have to do is rearrange the equation to solve for
We do this by dividing both sides by
Now all we have to do is plug in the given values:

