In a redox reaction, the substance that accepts electrons is said to be what?
1 Answer
Jul 19, 2016
An oxidizing agent.
Explanation:
As you know, a redox reaction involves the transfer of electrons from a chemical species that loses electrons to a chemical species that gain electrons.
The chemical species that loses electrons is undergoing oxidation and the chemical species that gains electrons is undergoing reduction.
As a consequence, the species that loses electrons acts as a reducing agent because its electrons are used to reduce another chemical species.
Similarly, the chemical species that gains electrons acts as an oxidation agent because it takes electrons, and thus it oxidizes, another chemical species.
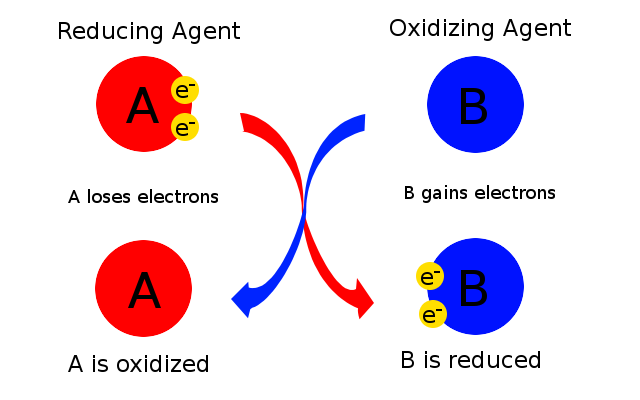
You can thus say that the chemical species that accepts electrons acts as an oxidizing agent.

