What is the number of valence electrons in nitrogen?
1 Answer
Nitrogen has
Explanation:
The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons.
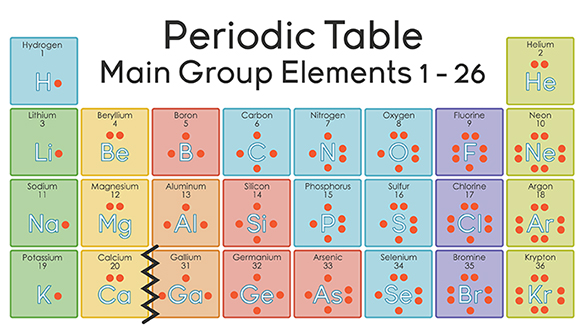
In your case, nitrogen,
As you know, nitrogen exists as diatomic molecules,
This is a direct consequence of the fact that each nitrogen atom has
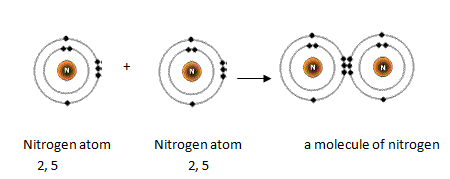
Another thing to mention here is the fact that nitrogen's

