If #2 L# of a gas at room temperature exerts a pressure of #72 kPa# on its container, what pressure will the gas exert if the container's volume changes to #7 L#?
1 Answer
Aug 3, 2016
The new pressure is
Explanation:
Let's begin by identifying our known and unknown variables.
The first volume we have is
The answer can be ascertained by using Boyle's Law:
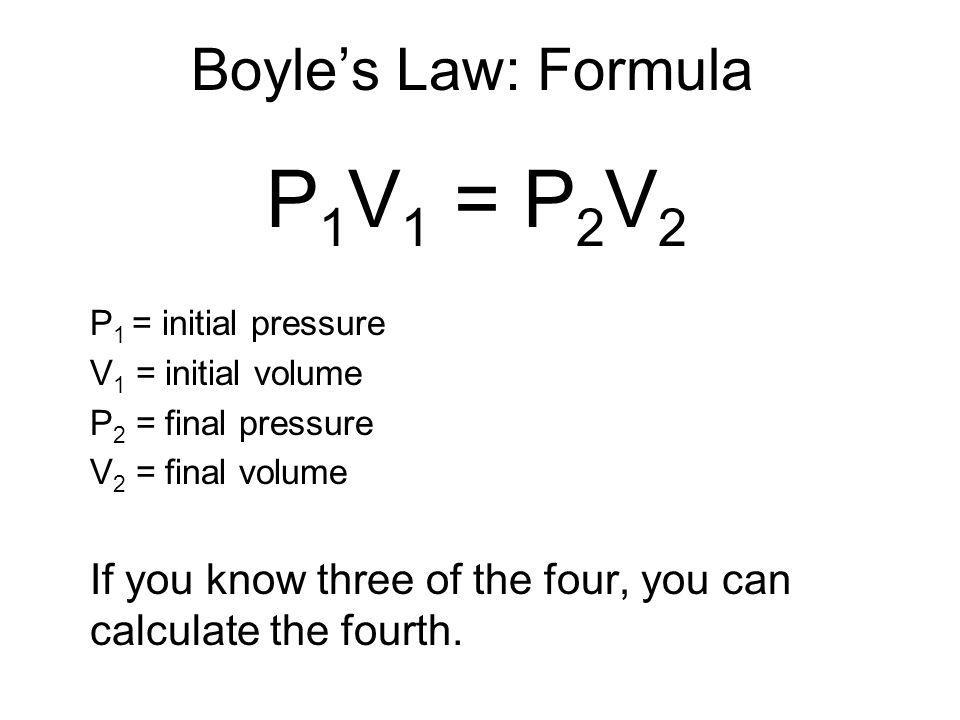
All we have to do is rearrange the equation to solve for the final pressure.
We do this by dividing both sides by
Plug in your given values to get your final pressure:

