What is the name of the compound that has the formula PCl3?
1 Answer
Aug 9, 2016
Phosphorus trichloride
Explanation:
When naming binary covalent compounds (compounds that are composed of 2 different nonmetals), you want to:
- Name the first element. You can obtain the name directly from the periodic table. The symbol, P, tells us that we are dealing with the element phosphorus
- Name the second element by using its root name and change the ending to -ide. The symbol, Cl, tells us that we have the element chlorine. So what you want to do is keep the root name (chlor) and change the -ine ending to -ide.
-
Then you add Greek prefixes to indicate the number of atoms that you have of that element:
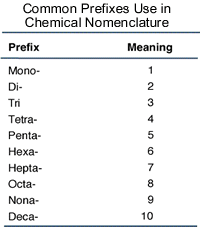 crescentok.com
crescentok.com -
The subscript that is in front of Cl tells us that we have 3 chlorine atoms, so we place the prefix (tri-) before the name of the second element.
Thus,
I hope this explanation makes sense!

