Question #044ba
1 Answer
Here's what I got.
Explanation:
Sodium metal,
#2"Na"_ ((s)) + "Cl"_ (2(g)) -> 2"NaCl"_ ((s))#
To check that this is indeed a redox reaction, assign oxidation numbers to the atoms that take part in the reaction
#2stackrel(color(blue)(0))("Na")_ ((s)) + stackrel(color(blue)(0))("Cl")_ (2(g)) -> 2stackrel(color(blue)(+1))("Na")stackrel(color(blue)(-1))("Cl")_ ((s))#
Notice that sodium metal goes from an oxidation state of
Similarly, chlorine goes from an oxidation state of
So, you know that sodium is being oxidized, which can only mean that chlorine is doing the oxidizing, i.e. chlorine acts as an oxidizing agent.
On the other hand, chlorine is being reduced, which can only mean that sodium is doing the reducing, i.e. sodium acts as a reducing agent.
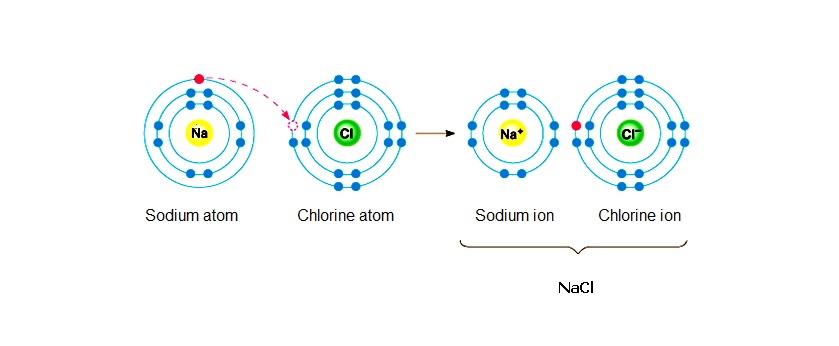
So, to sum this up, sodium acts as a reducing agent because it is giving its valence electron to a chlorine atom, thus reducing chlorine to chloride anions,
Chlorine acts as an oxidizing agent because it is taking away the valence electron of a sodium atom, thus oxidating sodium metal to sodium cations,

