What is the molar mass of heavy water #"D"_2"O"#?
1 Answer
Explanation:
Heavy water,
The difference between hydrogen-1 and deuterium is that the latter contains one proton and one neutron inside its nucleus, as opposed to the former which only contains a proton.
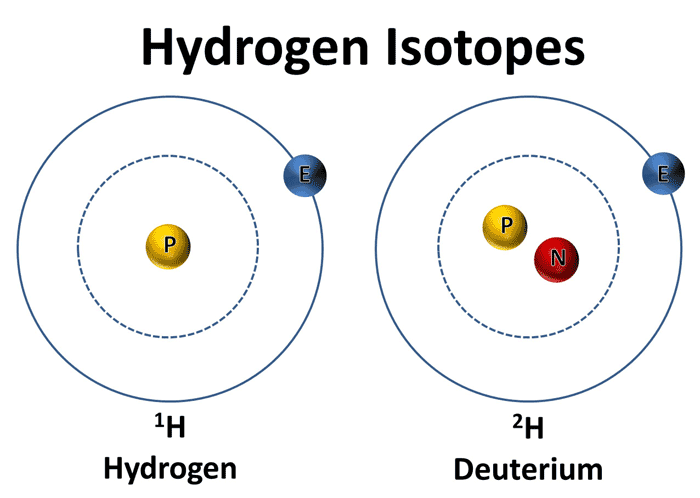
Now, deuterium has a molar mass of
#M_("M D") = "2.0141 g mol"^(-1)#
https://en.wikipedia.org/wiki/Deuterium
This means that the molar mass of heavy water, which contains two deuterium isotopes and one oxygen atom, will be equal to
#M_("M D"_2"O") = 2 xx "2.0141 g mol"^(-1) + "15.9994 g mol"^(-)#
#color(green)(|bar(ul(color(white)(a/a)color(black)(M_("M D"_2"O") = "20.0276 g mol"^(-1))color(white)(a/a)|)))#

