What is the number of valence electrons in oxygen?
1 Answer
Aug 25, 2016
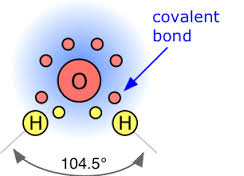
Oxygen is in Group VI, so there are 6 valence electrons in the oxygen ATOM.
Explanation:
For oxygen,
Two of these electrons are inner core, and are not conceived to participate in bonding. The remaining 6 are valence electrons, which participate in bonding and influence structure. Generally, 2 of these electrons combine with the electrons of donor atoms (cf hydrogens) to form covalent bonds. The remaining 4 valence electrons reside in stereochemically active lone pairs, which influence structure.