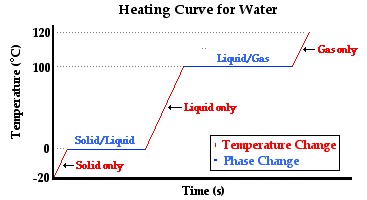
There are four separate heats involved in this problem:
- #q_1# = heat required to warm the ice from -11.09 °C to 0 °C
- #q_2# = heat required to melt the ice to water at 0 °C
- #q_3# = heat required to warm the water from 0 °C to 100 °C
- #q_4# = heat required to convert the water to steam at 100 °C
#q = q_1 + q_2 + q_3 + q_4 = nc_1ΔT_1 +nΔ_text(fus)H + nc_2ΔT_2 + nΔ_text(vap)H#
#bbq_1#
#n = "0.232 mol"#
#c_1 = "37.6 J·°C"^"-1""mol"^"-1"#
#ΔT = "0.0 °C - (-11.09 °C)" = "11.09 °C"#
#q_1 = ncΔT = 0.232 color(red)(cancel(color(black)("mol"))) × 37.6 color(white)(l)"J"·color(red)(cancel(color(black)( "°C"^"-1""mol"^"-1"))) × 11.09 color(red)(cancel(color(black)("°C"))) = "96.74 J" = "0.096 74 kJ"#
#bbq_2#
#Δ_"fus"H = "40.7 kJ·mol"^"-1"#
#q_2 = 0.232 color(red)(cancel(color(black)("mol"))) × 6.02color(white)(l) "kJ"·color(red)(cancel(color(black)("mol"^"-1"))) = "1.397 kJ"#
#bbq_3#
#c_2 = "75.2 J·°C"^"-1""mol""-1"#
#ΔT_2 = "100 °C - 0°C" = "100 °C"#
#q_3 = ncΔT = 0.232 color(red)(cancel(color(black)(" mol"))) × 75.2 color(white)(l)"J"·color(red)(cancel(color(black)( "°C"^"-1""mol"^"-1"))) × 100 color(red)(cancel(color(black)("°C"))) = "1745 J" = "1.745 kJ"#
#bbq_4#
#Δ_"vap"H = "40.7 J·K"^"-1""mol""-1"#
#q_4 = nΔ_"vap"H = 0.232 color(red)(cancel(color(black)("mol"))) × 40.7color(white)(l) "kJ"·color(red)(cancel(color(black)("mol"^"-1"))) = "9.442 kJ"#
#q = q_1 + q_2 + q_3 + q_4 = "0.096 74 kJ" + "1.397 kJ" + "1.745 kJ" + "9.442 kJ" = "12.68 kJ"#


