How many core electrons are in a ground state atom of arsenic?
1 Answer
28 Core Electrons.
Explanation:
Arsenic (As) has an atomic number of 33 (according to the periodic table). This means that an As Atom in the ground state (not ionized) should have 33 electrons.
So, let's quickly define core and valence electrons.
Valence: Electrons in the top ward energy level, ones that participate in bonding with other atoms.
Core: Electrons that are shielded from bonding by the Valence Electrons.
Think of the beach ball. The outward plastic skin on the ball would be the valence electrons. The valence electrons are what touch your hands and touch the air. The air on the inside of the ball are the core electrons. They don't touch anything since your hand and the outside air touches the plastic and is shielded.
The simplest way to find out how many valence electrons an element has is by using the group. A group is the column in the periodic table. If we refer to the periodic table below:
Table 1:
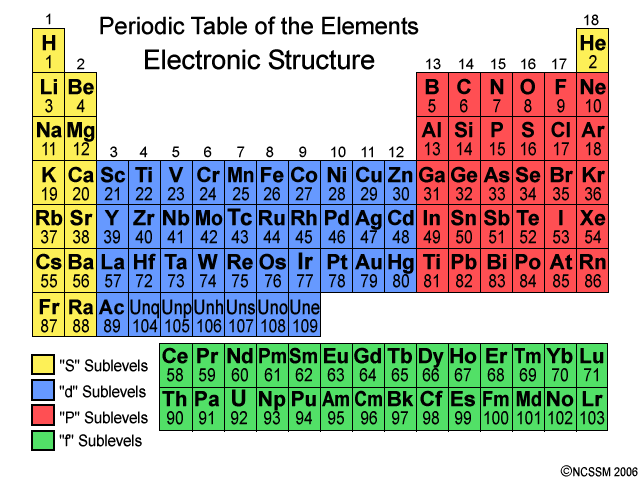
We will see that Arsenic (33e-) is in Group 15, since it is in the 15th vertical row. Then, we can consult this chart below to find out how many valence electrons it has.
Group 1: 1 Ve-
Group 2: 2 Ve-
Group 13: 3 Ve-
Group 14: 4 Ve-
Group 15: 5 Ve-
Group 16: 6 Ve-
Group 17: 7 Ve-
Group 18: 8 Ve- (except for helium, which has 2)
So, now we know that since it is in Group 15, it must have 5 valence electrons (Ve-).
To find the amount of core electrons, follow this formula.
Core Electrons = Total Electrons - Valence Electrons
So, that'd be:
28 Core Electrons.
Cheers!

