If #9/4 L# of a gas at room temperature exerts a pressure of #16 kPa# on its container, what pressure will the gas exert if the container's volume changes to #9/8 L#?
1 Answer
Sep 27, 2016
The final pressure will be 32 kPa.
Explanation:
This is a problem involving Boyle's law, which states that the volume of a gas at constant temperature and amount, is inversely proportional to the pressure. This means that when the volume increases, the pressure will decrease.
The equation for solving this kind of problem is:
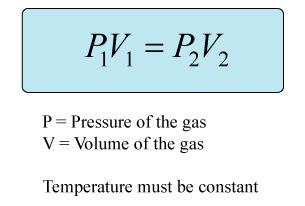
Known
Unknown
Rearrange the equation to isolate
Note: Notice that the volume decreased by half, and the volume increased 2x.

