Using the phase diagram for #H_2O#, what phase is water in at 1 atm pressure and -5 C?
1 Answer
Sep 28, 2016
Clearly water is a solid phase under these circumstances.
Explanation:
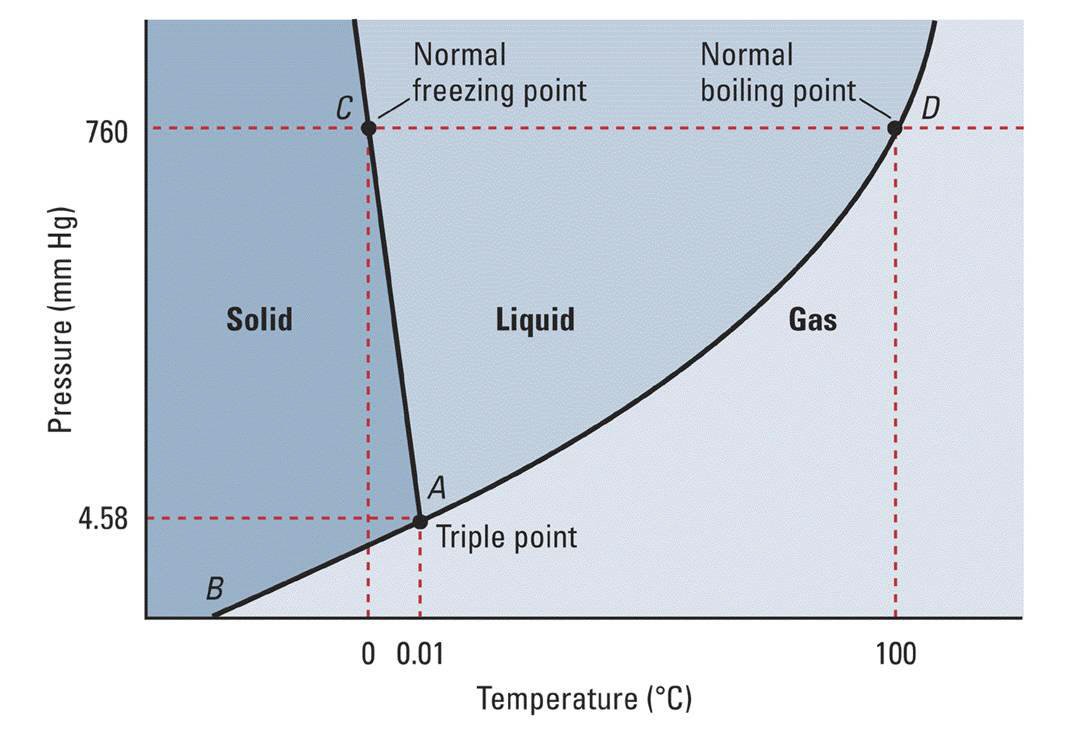
The phase diagram plots pressure (in

