Spectator ions are those which are in solution but do not react. Are there any spectator ions for the reaction of sodium phosphate with calcium bromide?
1 Answer
Yes, there are.
Explanation:
In order to see whether or not you'll have spectator ions when this reaction takes place, examine the solubility of the chemical species involved in the reaction.
To do that, use the solubility rules for ionic compounds.
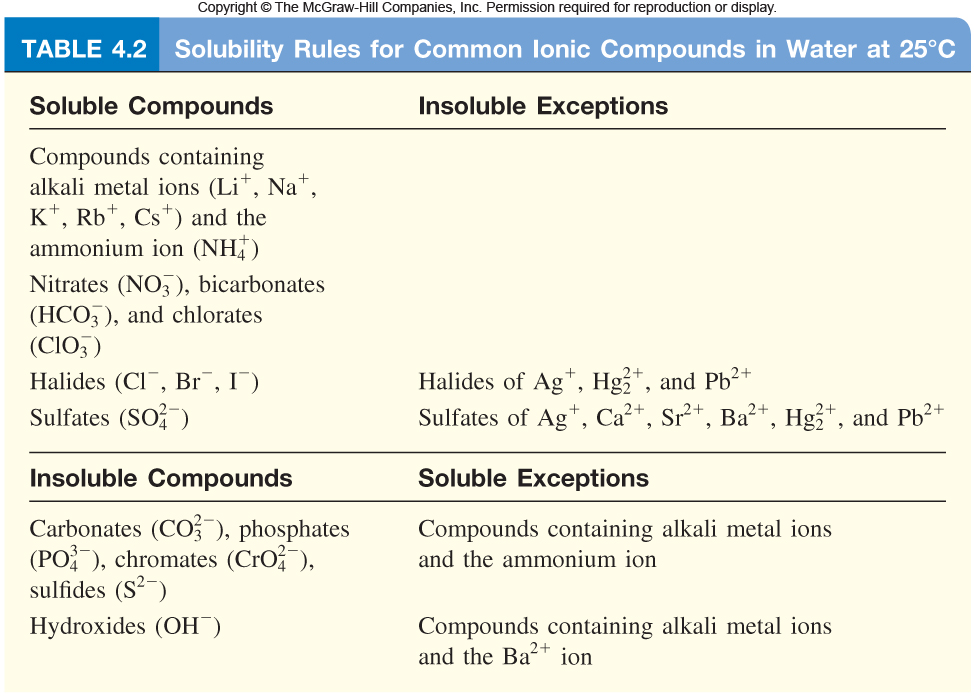
Sodium phosphate,
#"Na"_ 3"PO"_ (4(aq)) -> 3"Na"_ ((aq))^(+) + "PO"_ (4(aq))^(3-)#
The same can be said of calcium bromide,
#"CaBr"_ (2(aq)) -> "Ca"_ ((aq))^(2+) + 2"Br"_ ((aq))^(-)#
Now, when you mix these two solutions, you will end up with calcium phosphate,
As you can see from the table, calcium phosphate is insoluble in aqueous solution. This means that when the calcium cations combine with the phosphate anions, a solid will precipitate out of solution.
On the other hand, sodium bromide is soluble in aqueous solution, which implies that it exists as ions in solution.
This means that the sodium cations,
#2 xx [3"Na"_ ((aq))^(+) + "PO"_ (4(aq))^(3-)] + 3 xx ["Ca"_ ((aq))^(2+) + 2"Br"_ ((aq))^(-)] -> "Ca"_ 3("PO"_ 4)_ (2(s)) darr + 6"Na"_ ((aq))^(+) + 6"Br"_ ((aq))^(-)#
This is equivalent to
# color(blue)(6"Na"_ ((aq))^(+)) + 2"PO"_ (4(aq))^(3-) + 3"Ca"_ ((aq))^(2+) + color(purple)(6"Br"_ ((aq))^(-)) -> "Ca"_ 3("PO"_ 4)_ (2(s)) darr + color(blue)(6"Na"_ ((aq))^(+)) + color(purple)(6"Br"_ ((aq))^(-))#
Removing the spectator ions will get you the net ionic equation
#3"Ca"_ ((aq))^(2+) + 2"PO"_ (4(aq))^(3-) -> "Ca"_ 3("PO"_ 4)_ (2(s)) darr#

