Question #784d6
1 Answer
All of the above.
Explanation:
All you really have to do here is be familiar with the solubility rules for ionic compounds.
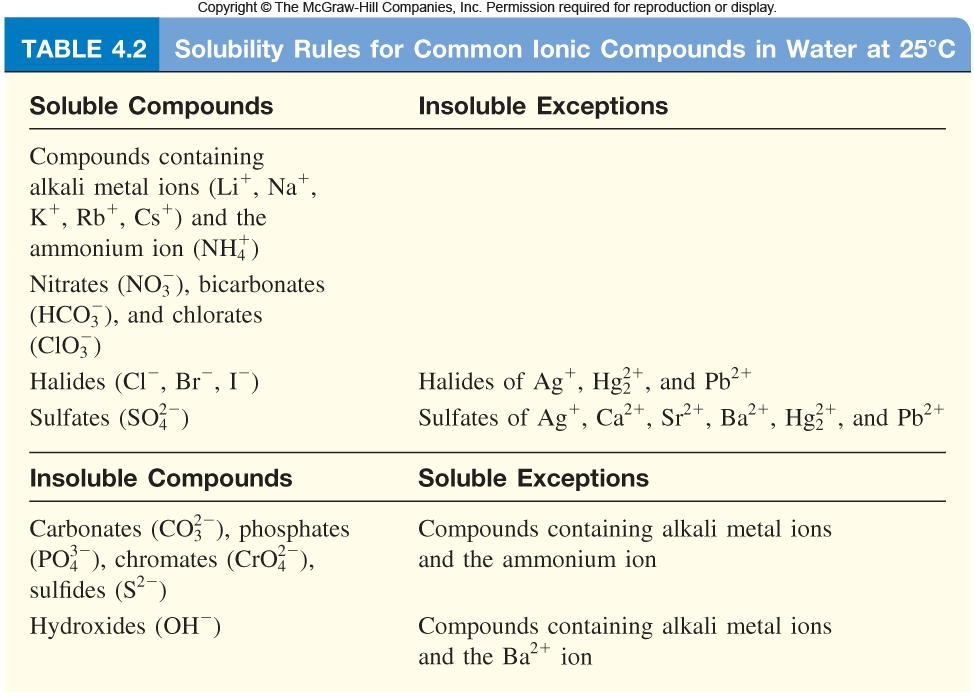
Lead(II) sulfate,
The same can be said of silver chloride,
The silver cations,
There's really no workaround knowing the solubility rules, so make sure that you're at least familiar with some of the more important soluble and insoluble ionic compounds.

