Which of the following elements has the lowest electronegativity? How do you know?
a. lithium
b. carbon
c. bromine
d. fluorine
a. lithium
b. carbon
c. bromine
d. fluorine
1 Answer
Lithium
Explanation:
Electronegativity is the tendency for an atom to attract electrons. There are general trends on the periodic table which you can use to determine the relative electronegativity for different elements, but otherwise you can also look at their valence shell and effective nuclear charge to determine their relative electronegativity.
In the valence shells of lithium, carbon, bromine and fluorine, lithium is the only element where it is more disadvantageous to gain electrons instead of losing electrons as to gain a "full outer shell"
Lithium:
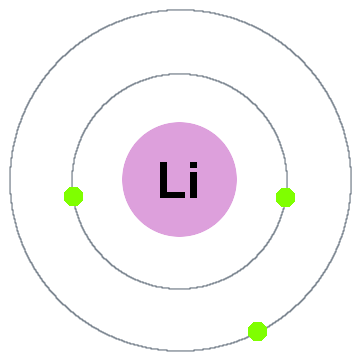
As such, it has a lower tendency to attract electrons , and thus has a lower electronegativity. In the case of carbon, bromine and fluorine, they all can gain electrons to achieve "full outer shells" (fluorine is in fact the most electronegative atom).
Trends of electronegativity:
)
- Note that this excludes the noble gases.

