You are asked to make a buffer solution with a pH of 2.0. Use the information below to identify an appropriate choice of an acid and a conjugate base to make the buffer?
You are asked to make a buffer solution with a pH of 2.0. Use the
information below to identify an appropriate
choice of an acid and a conjugate base to make the buffer. Specify both the acid
and the conjugate base you would use. (Hint: There may be more than one right
answer to this problem.)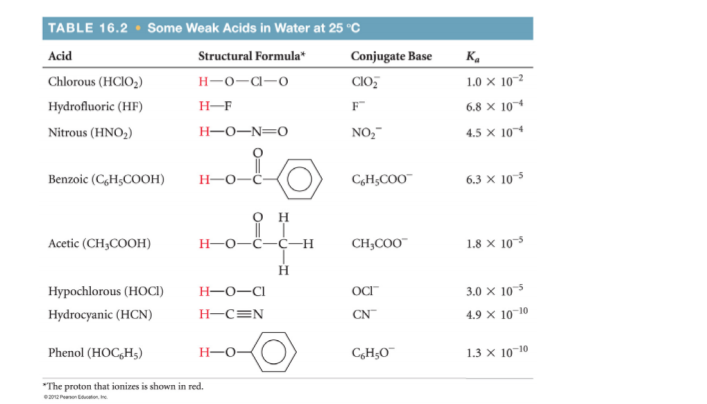
You are asked to make a buffer solution with a pH of 2.0. Use the
information below to identify an appropriate
choice of an acid and a conjugate base to make the buffer. Specify both the acid
and the conjugate base you would use. (Hint: There may be more than one right
answer to this problem.)
1 Answer
There is no simple answer to this question.
Explanation:
It is tempting to say that we should use a chlorous acid buffer, because the
We could then use the Henderson-Hasselbalch equation to calculate the required ratio of the acid to its conjugate base.
However, the predictions of the Henderson-Hasselbalch equation can deviate significantly from the actual values when
We would have to do exact calculations using the simultaneous equilibria involved.
That is probably beyond the scope of this question.

