Question #e6edb
1 Answer
Explanation:
Your strategy here will be to draw the Lewis structure of xenon oxytetrafluoride,
Start by calculating the total number of valence electrons you have in xenon oxytetrafluoride
- xenon contributes eight valence electrons
- oxygen contributes six valence electrons
- each of the four fluoride atoms contributes seven valence electrons
The total number of valence electrons will be
#"8 e"^(-) + "6 e"^(-) + 4 xx "7 e"^(-) = "42 e"^(-)#
Now, the xenon atom will be the central atom of the molecule. It will form a double bond with the oxygen atom and a single bond with each of the four fluorine atoms.
These bonds will account for
#overbrace(1 xx "4 e"^(-))^(color(red)("one double bond")) + overbrace(4 xx "2 e"^(-))^(color(blue)("four single bonds")) = "12 e"^(-)#
These electrons act as bonding electrons because they are used by the atoms to form covalent bonds.
The remaining number of electrons will be distributed as lone pairs
#overbrace("42 e"^(-))^(color(purple)("total no. of valence electrons")) - overbrace("12 e"^(-))^(color(brown)("bonding electrons")) = overbrace("30 e"^(-))^(color(darkgreen)("in lone pairs"))#
Since each lone pair is composed of two electrons, the total number of lone pairs present in a xenon oxytetrafluoride molecule will be
#color(green)(bar(ul(|color(white)(a/a)color(black)("no. of lone pairs" = "30 e"^(-)/2 = "15 lone pairs")color(white)(a/a)|)))#
Now, these lone pairs will be distributed as follows
- three lone pairs on each of the four fluorine atoms
- two lone pairs on the oxygen atom
- one lone pair on the xenon atom
The Lewis structure of xenon oxytetrafluoride looks like this
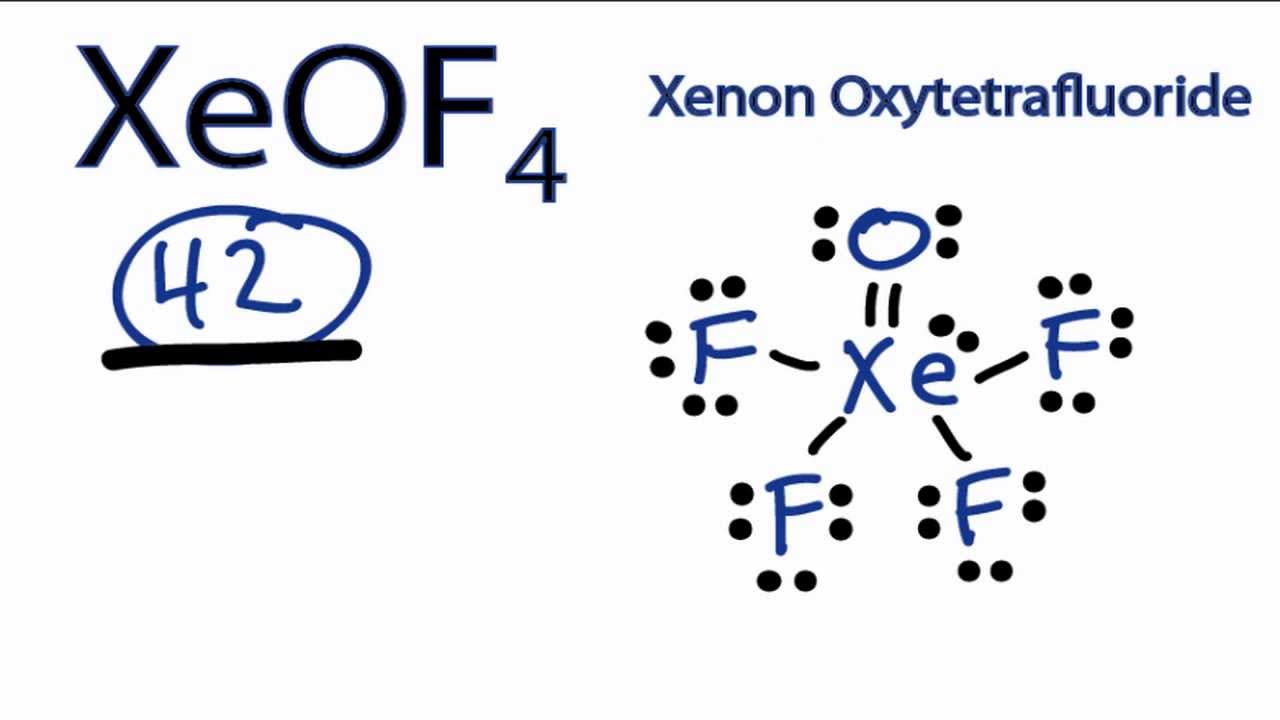
As you can see, the
Notice that xenon has a total of

