In calorimetry, we often define #q_(sys) = 0#. What assumptions about the calorimeter make this possible?
1 Answer
Oct 25, 2016
If you mean with respect to the surroundings, then it must mean that heat cannot flow in from the surroundings,
The walls of the container must therefore be impermeable and insulated. In such a calorimeter,
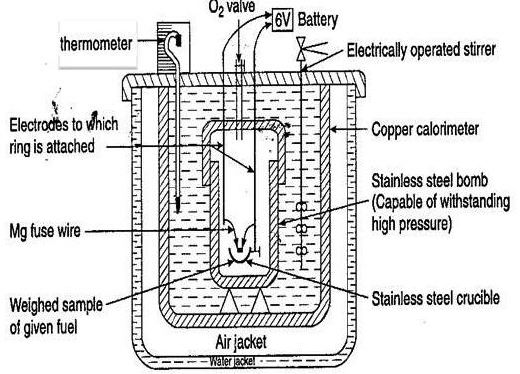
Overall, the calorimeter would have to be closed, with impermeable, insulated walls.

