How do water molecules act like "little magnets"?
1 Answer
Nov 3, 2016
Water molecules are polar in nature.
Explanation:
Water molecules are basically,
Thus a polarity develops in each
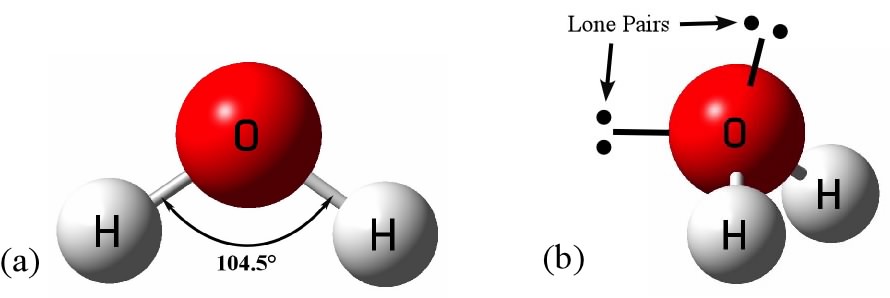 Bent shape of water
Bent shape of water
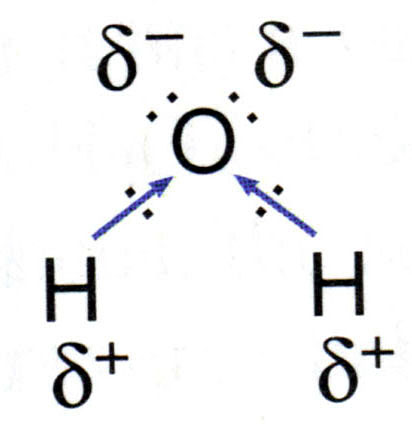 Polar water molecule
Polar water molecule

