What are the oxidation states of carbon in acetic acid?
2 Answers
Oxidation states don't really mean much in covalent compounds like acetic acid. In here, there are two different carbons, each with their own oxidation state.
If you want to just do it, then assume
#[stackrel(0)("C"_2)stackrel(+1)("H"_3)stackrel(-2)("O"_2)]^(-)#
Then you should find that the two carbons' oxidation states must somehow add to be
#stackrel(+1)("H"_3)stackrel(color(blue)(-3))("C")-#
#-stackrel(color(blue)(+3))("C")stackrel(-2)("O")stackrel(-2)("O")stackrel(+1)("H")#
And so we still have
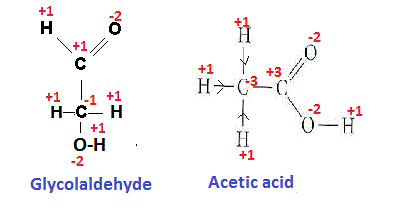
Here the two possible structural formula have been derived from given molecular formula
Oxidation states (os) of different elements have been shown in the figure.
The oxidation states of different atoms in the covalent compound are calculated on the basis of the assumption that the molecule is an aggregate of ions and gain and loss of electron/s between bonded atoms occur depending on their electronegativity difference.
In acetic acid
C-atom of >C=O group gives two e to one O atom linked with it through double bond and also gives one e to the O atom of -OH group linked with it with through single bond and no electron is gained from C atom linked with it. So net loss of e is 3 and OS of it becomes +3.
But in
Similarly we can calculate the oxidation states of different atoms in the Glycolaldehyde molecule (having molecular formula


