Question #a20b5
1 Answer
The solution is basic.
Explanation:
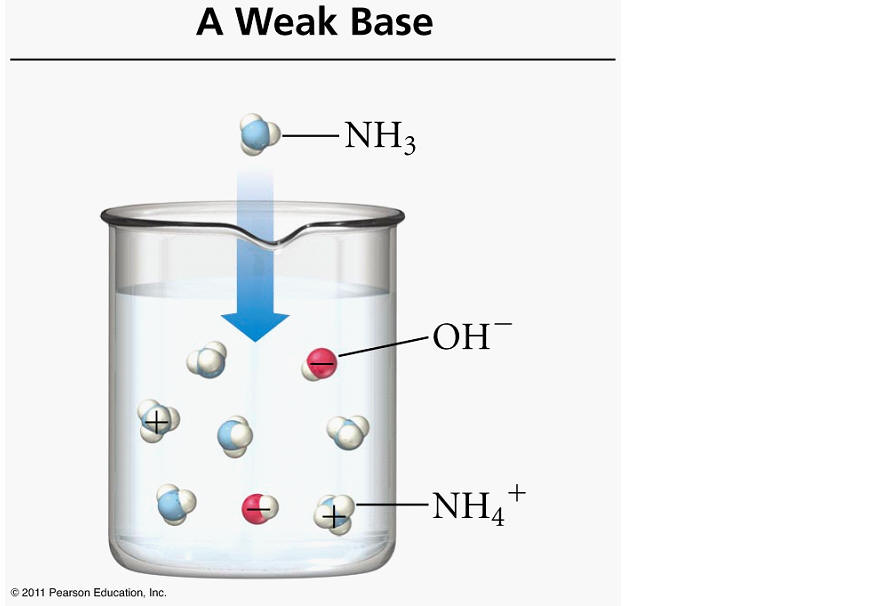
Take a look at the balanced chemical equation given to you
#"NH"_ (3(g)) + "H"_ 2"O"_ ((l)) rightleftharpoons "NH"_ (4(aq))^(+) + "OH"_ ((aq))^(-)#
Notice that the reaction produces hydroxide anions,
It's true that the ammonium cation,
So, the reaction produces hydroxide anions, which in turn decrease the concentration of hydronium cations and increase the pH of the solution.
Ammonia is considered a weak base because it does not ionize completely to produce hydroxide anions, i.e. the majority of the ammonia molecules dissolved in aqueous solution will not ionize.