My teacher says the correct answer is 7, but I don't understand why. Can anyone explain?
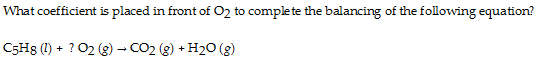
1 Answer
Explanation:
Chemical reactions conserve mass and charge. What does this mean? It means that if there are
And are there
If you don't see this immediately, voice your objection, and someone here will make another attempt. The idea of stoichiometry, of mass equivalence, is fundamental to the study of chemistry. (It is fundamental to accounting also! Why?) As a start count up how many carbon, hydrogen, and oxygen atoms on the left hand side of the equation, and compare them to the number of
Here is a similar question on redox equations. Once again, it is required to balance both mass and charge on each side of the equation. See here for a similar answer on stoichiometry.

