What is the purpose of adding Zn or Al in a blue Copper (#CuSO_4#) solution?
1 Answer
The purpose is to compare the reactivity of aluminum and zinc to copper. If the aluminum and zinc are more reactive than copper, each will undergo a single replacement reaction with copper sulfate, producing the metal copper as a product.
Explanation:
You are observing whether a single replacement (single displacement) reaction will take place between the elements Zn or Al by adding each to a copper(II) sulfate solution.
The following illustration indicates what happens when a single replacement reaction occurs involving metals.
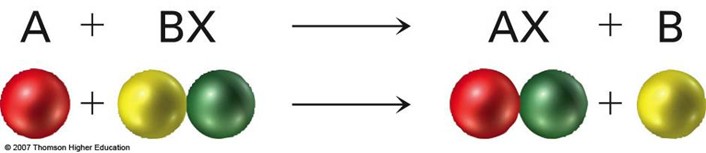
We can determine whether a single replacement will take place by comparing the locations of the metals on a metal activity series, or list. A metal on the list can replace any of the metals below it.
The following is a metal activity series.
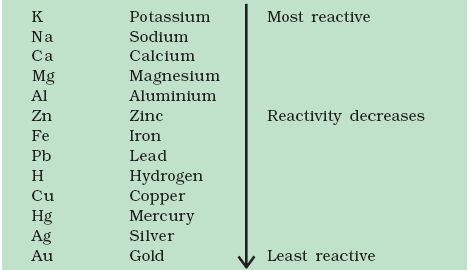
As you can see, aluminum and zinc are above copper on the activity series, so the aluminum and zinc will replace the copper in the copper sulfate solution.

