The gas inside of a container exerts #15 Pa# of pressure and is at a temperature of #640 ^o K#. If the pressure in the container changes to #36 Pa# with no change in the container's volume, what is the new temperature of the gas?
1 Answer
Nov 14, 2016
The final temperature will be 1500 K.
Explanation:
This question is an example of Guy-Lussac's law which states that the pressure of a given amount of gas held at constant volume, is directly proportional to its Kelvin temperature.
The equation to use is:
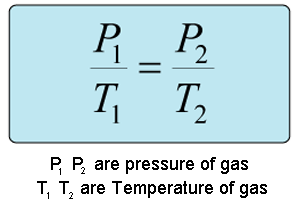
Known
Unknown
Solution
Rearrange the equation to isolate

