What is the velocity of an electron that makes a transition corresponding to the longest wavelength line of the Lyman series?
2 Answers
Explanation:
Energy of photon
Since
Finding wavelength
Here value of
Therefore wavelength
Energy of photon must be equal to energy of electron
After solving velocity
or velocity =
Explanation:
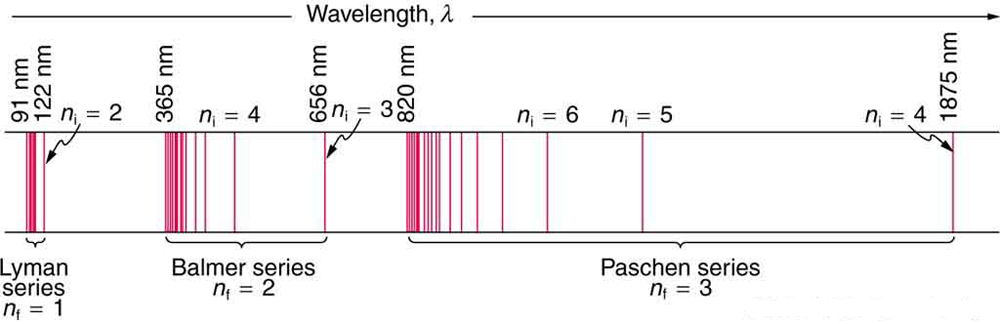
The Lyman series corresponds to the emission lines of a hydrogen atom that has an electron make a transition to the lowest energy level of the electron, which, of course, corresponds to
The trick here is to realize the fact that as an electron moves from an increasingly higher energy level to
Simply put, the bigger the difference between
#color(blue)(ul(color(black)(E = h * nu))) -># the Planck - Einstein relation
with
#E# - the energy of the photon#h# - Planck's constant, equal to#6.626 * 10^(-34)"J s"# #nu# - the frequency of the photon
Since frequency and wavelength have an inverse relationship as given by
#color(blue)(ul(color(black)(lamda * nu = c)))#
with
#c# - the speed of light in a vacuum, equal to#"299,792,458 m s"^(-1)#
you can say that the energy of the photon is inversely proportional to its wavelength. In other words, the longer the wavelength, the less energetic the photon.
This implies that the longest wavelength line in the Lyman series will correspond to the lowest energy emission. Therefore, you can say that
#n=2 -> n=1# The lowest energy emission corresponds to the longest wavelength
Now all you have to do is use the Rydberg equation to find the needed wavelength.
#color(blue)(ul(color(black)(1/(lamda) = R_(oo) * (1/n_f^2 - 1/n_i^2)#
with
#R_(oo) ~~ 1.097373 * 10^7"m"^(-1)# #n_f = 1# #n_i = 2#
Rearrange to solve for
#lamda = 1/(R_(oo) * (1 - 1/n_i^2))#
Plug in your values to find
#lamda = 1/(1.097373 * 10^(7)"m"^(-1) * (1 - 1/4)) = 1.215 * 10^(-7)"m"#
This corresponds to the actual value of the wavelength, which is equal to approxiamtely
Now, in order to find the velocity of the electron, you must use the fact that its kinetic energy must equal the energy of the emitted photon.
The kinetic energy lost by the electron while making a transition from a higher energy level to a lower energy level is equal to the energy of the emitted photon.
#E_"photon" = E_"kinetic"#
This will get you
#h * nu = 1/2 * m * v^2#
Use the wavelength of the photon to write
# h * c/(lamda) = 1/2 * m * v^2#
The mass of the electron in kilograms is
#m ~~ 9.1094 * 10^(-31) "kg"#
Rearrange the above equation to solve for
#v^2 = (2 * h * c)/(lamda * m) implies v = sqrt((2 * h * c)/(lamda * m))#
Plug in your values to find -- keep in mind that you have
#"1 J" = "1 kg m"^2 "s"^(-2)#
and so the units for Planck's constant are
#"J" * "s" = "kg m"^2"s"^(-2) * "s" = "kg m"^2"s"^(-1)#
#v = sqrt( (2 * 6.626 * 10^(-34) color(red)(cancel(color(black)("kg"))) "m"^2"s"^(-1) * 299,792,458 color(red)(cancel(color(black)("m"))) "s"^(-1))/(1.215 * 10^(-7)color(red)(cancel(color(black)("m"))) * 9.1094 * 10^(-31)color(red)(cancel(color(black)("kg")))))#
#v = "1,894,602.2 m s"^(-1)#
Rounded to four sig figs and expressed in scientific notation, the answer will be
#v = color(darkgreen)(ul(color(black)(1.895 * 10^6"m s"^(-1))))#


