Question #f99b8
1 Answer
Nov 24, 2016
Carbon dioxide has three lewis structures in total.
Explanation:
Carbon dioxide has a lewis structure where the
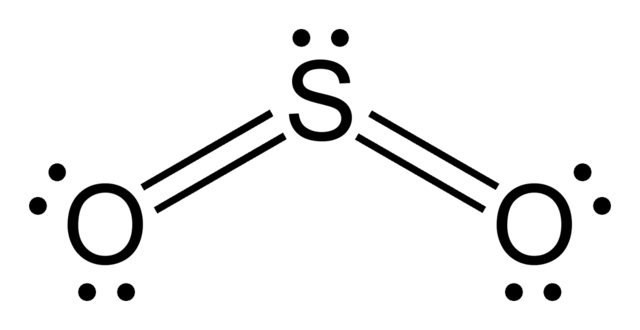
But, it has two more resonating structures formed by the shifting of the pi bonds.
The two most common cannonical structures of
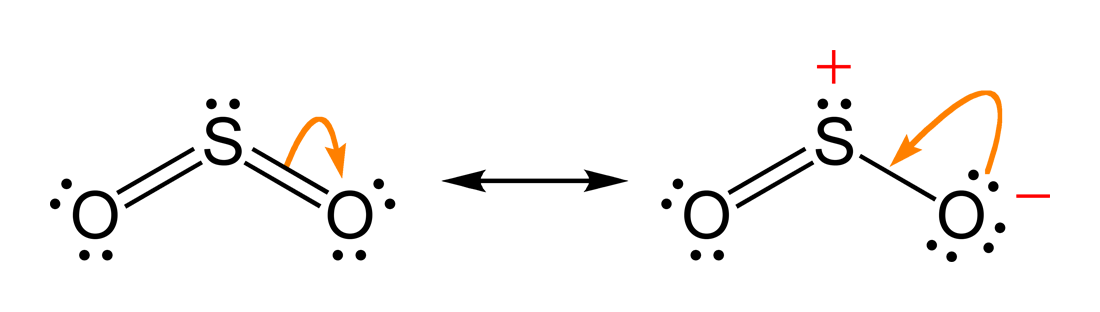
So,
Hope it Helps :)

