How do isotopes differ in atomic structure?
2 Answers
Nov 27, 2016
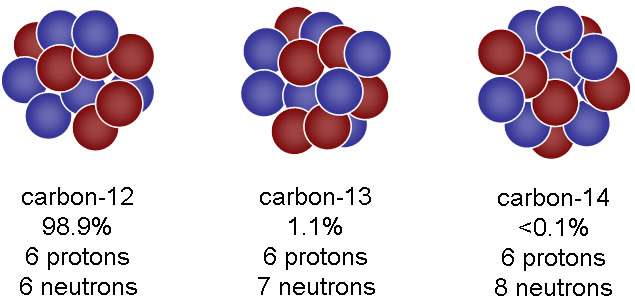
The nucleus differs as a result of the number of neutrons in the atom.
Explanation:
The actual structure doesn't differ all that much, but its mass does differ, thus its density is a little different. This is the result of the different number of neutrons that are located in the "center" of the atom.
For example (the image below), carbon has three isotopes, each with a different number of neutrons. As you can see the physical structures don't aesthetically appear too different from each other, but rather their densities differ a little bit.
Nov 27, 2016


