How does an atom's valence electron configuration determine its place in the periodic table?
1 Answer
Nov 28, 2016
Elements in a family (groups, the vertical bits) have the same number of valence electrons. The level of energy of an atom's valence electron(s) determines its period, (the horizontal lines).
Explanation:
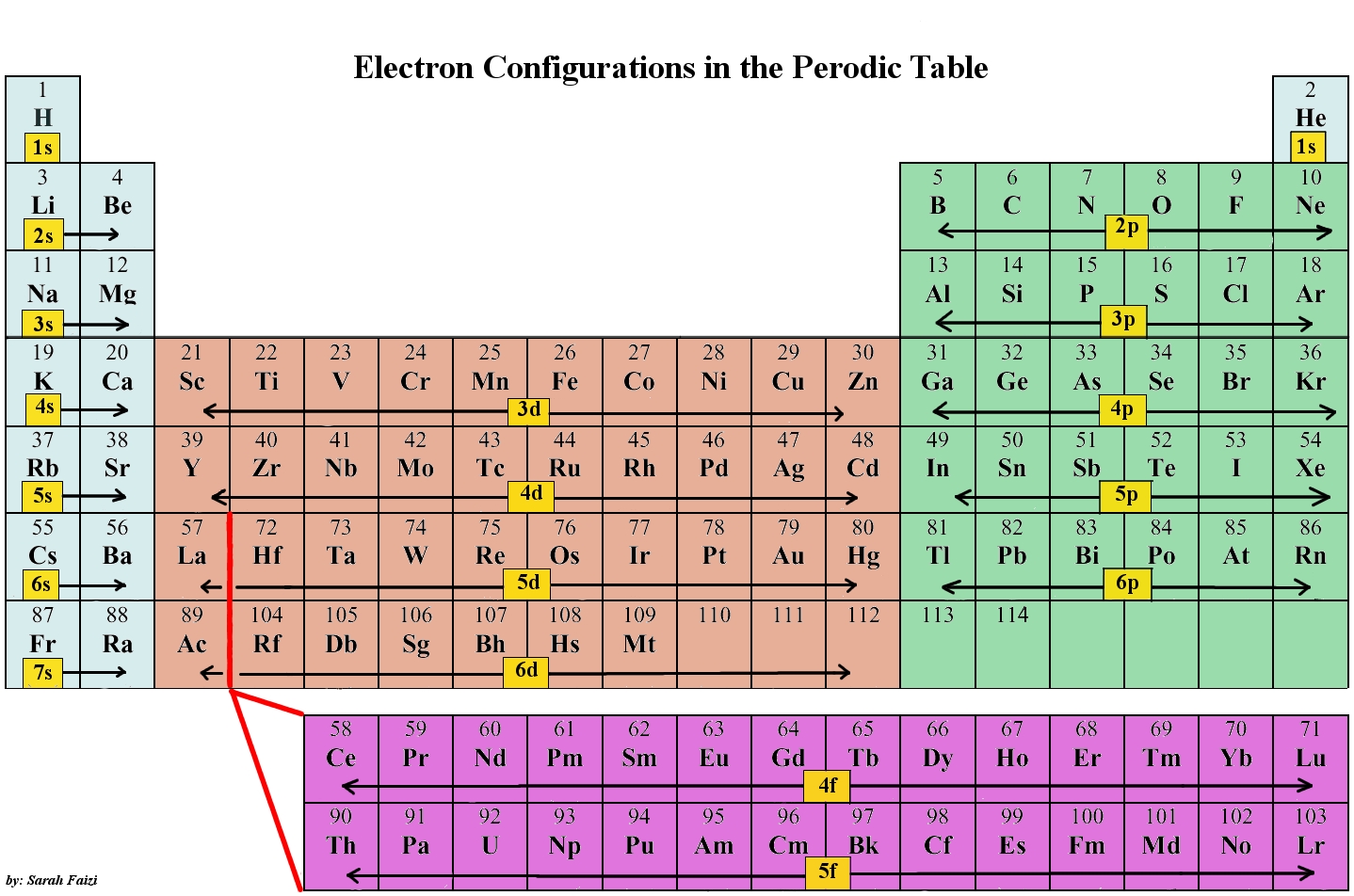
When you're looking for the element, try to fill in from what you're given.
E.g: Let's say you're working with Aluminium(Al),
That configuration is
See how it filling in across until it reached Aluminium?
Now, if you had
You would get Co, Cobalt.
Hope this helps.

