What happens to electrons when a polar covalent bond forms?
1 Answer
Dec 5, 2016
They are unequally shared about the species.
Explanation:
Consider the diagram of
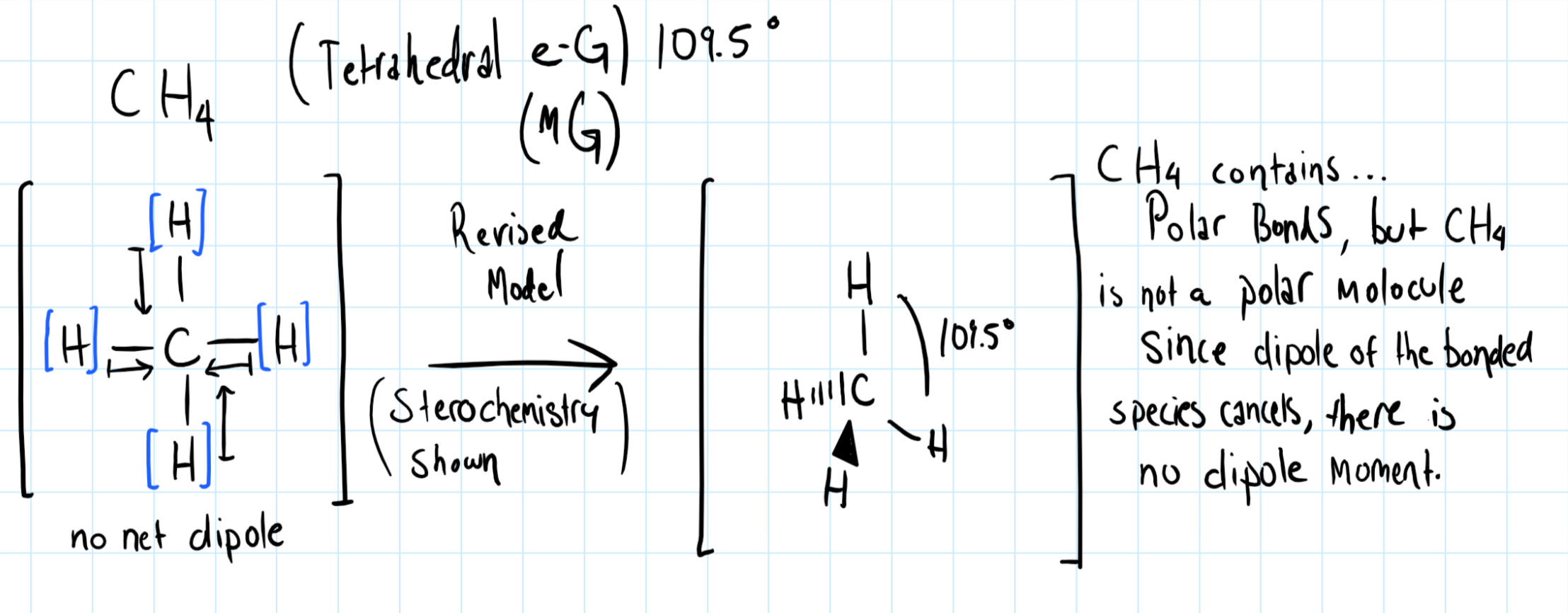
In the C-H bonds, the electron are shared unequally between Carbon and Hydrogen. The dipole points toward Carbon because the C-H bond is polar covalent, and this is because Carbon is more electronegative than Hydrogen.
However in

