What does the sign #uarr darr# represent with respect to electrons?
2 Answers
Spin up or spin down about the orbital (up arrow is spin up, down arrow is spin down)
Explanation:
Specifically, it is deonted by the
Remember that each electron has its own set of quantum number, consider the graphic below which shows quantum number notation.
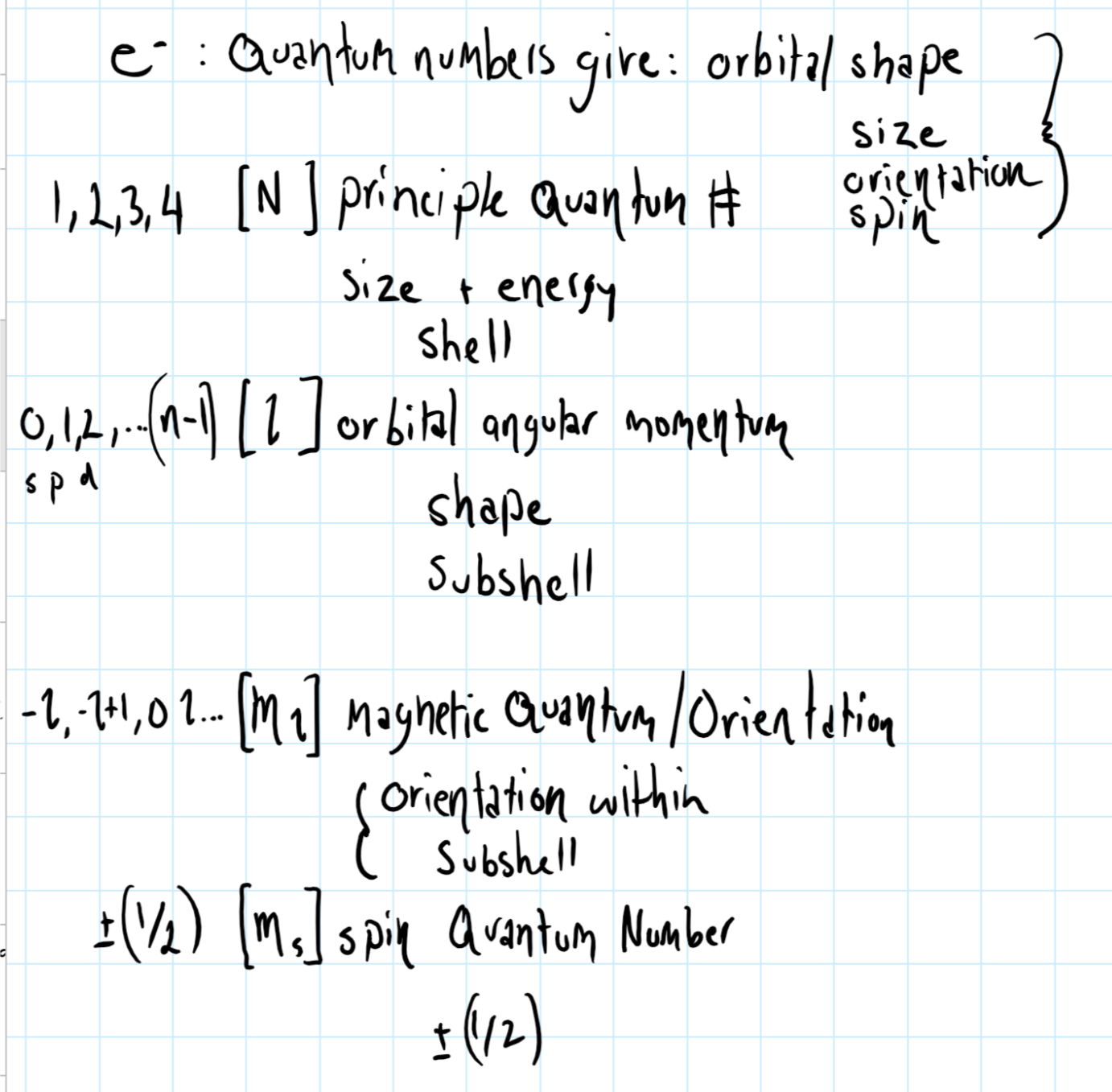
The arrows give the orientation of a characteristic of electrons (and many other particles for that matter) known as spin .
Explanation:
Every electron in an atom must be unique in terms of four characteristics. This is known as the Pauli Exclusion Principle and is fundamental to quantum mechanics.
These characteristics are given by the four quantum numbers assigned to each electron during the solution of Schroedinger's equation -
The first three of these determine the shell, subshell and orbital (respectively) in which the electron is located. The fourth, the spin quantum number can only have two values
Unfortunately there is no property in the "big" world we live in that corresponds to spin, and so it is a difficult quantity to grasp.
The unfortunate(?) choice for the name of this quantity has led many people to think that electrons really spin in some way. They do not. Spin does however result in a small magnetic character for an electron that is similar to what would be caused by a circulating charge so the name is not all bad!



