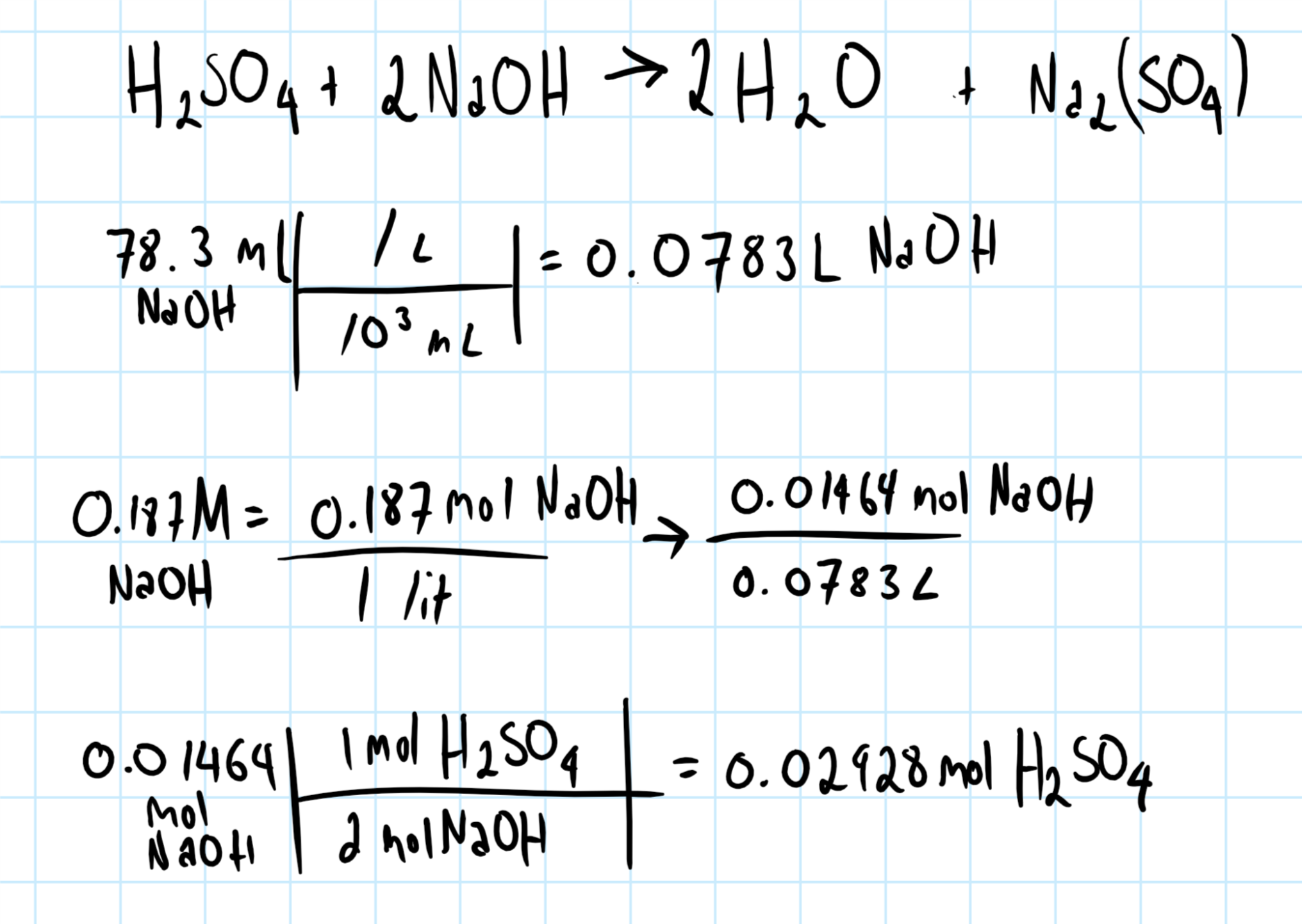How many mols of #H_2SO_4# will neutralize 78.3 mK of 0.187 N #NaOH#?
1 Answer
Dec 21, 2016
See below.
Explanation:
For this calculation, I'm assuming you meant 78.3 mL (not "mK") and 0.187M NaOH (basically that you made two major typos, since both K and N are close together to letters l and M which would make sense on a standardized keyboard).
If that is the case, and the units are not arbitrary, then the calculation should be correct. If it's not then I'm sorry as this is the best I could do with the information provided.
If this is the type of question you are trying to answer, remember to always start with a balanced chemical equation. The chemical system in question must be balanced to perform the calculation.
In our case:
and
Consider the graphic below: