In terms of electron affinity, can you explain this statement: "the stronger the attraction, the more energy is released"?
1 Answer
Electron affinity is basically how the energy of the atom changes due to the addition of a new electron.
So, I like to think of it as "how much might this new electron stabilize the atom, if it does?"
If a lot, then the electron affinity should be very negative, and the atom is very attracted to this new electron, releasing energy to stabilize itself as it acquires this new electron, lowering its ground-state energy.
Otherwise, the electron affinity is small and negative (such as for boron), or maybe even positive (such as for the noble gases).
Therefore, the statement, "the stronger the attraction, the more energy is released", can be paraphrased without loss of meaning to say:
"The more the electron can stabilize the atom, the more negative the electron affinity is."
Take a look at this chart here for some data, so you can verify that
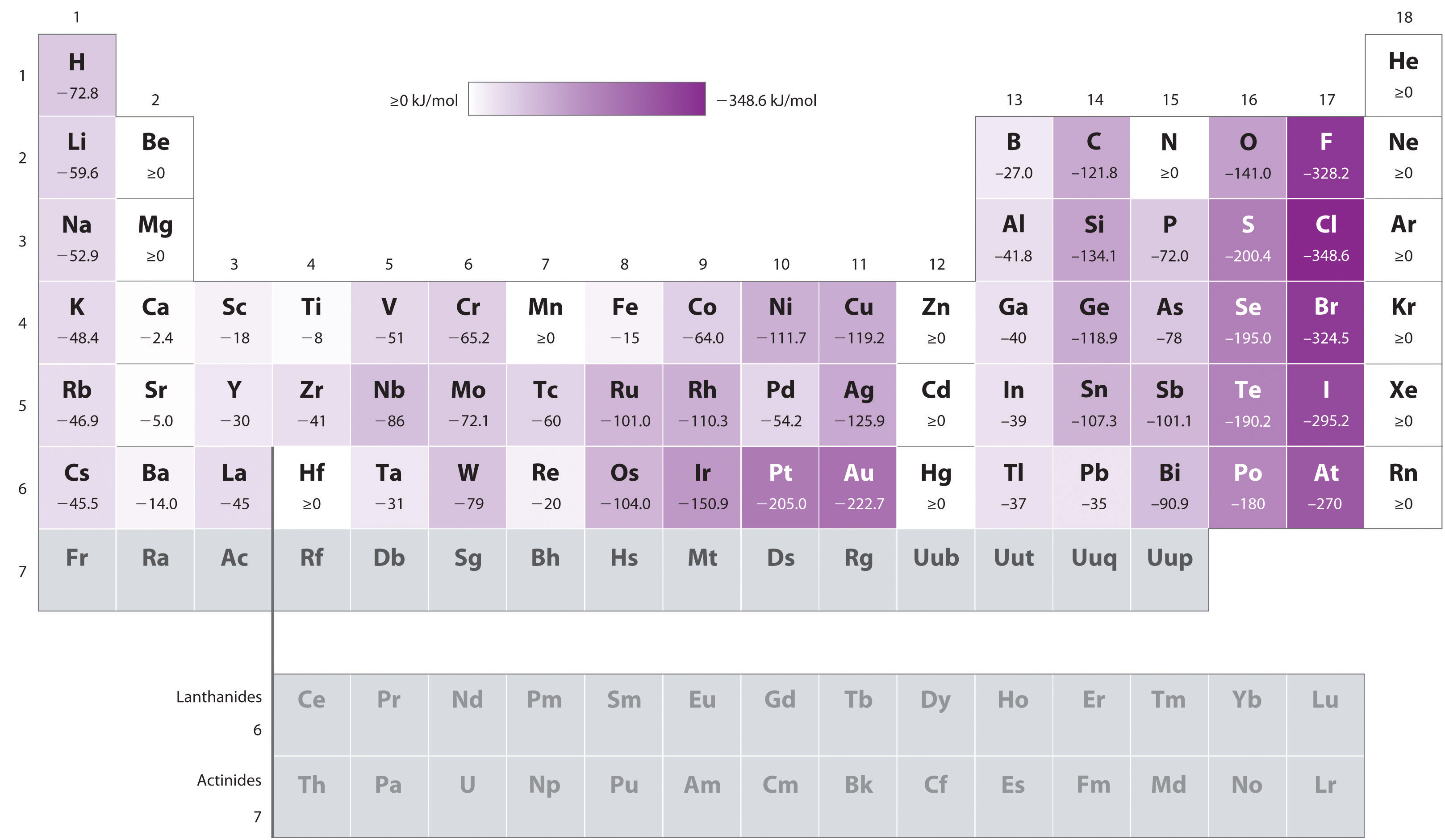 http://2012books.lardbucket.org/
http://2012books.lardbucket.org/
The numbers show that for instance, halogens follow this process for electron affinity (where
"X" + e^(-) -> "X"^(-) + Delta
This resembles an exothermic reaction, where energy is released.

