How many electrons in an atom can have the n = 5, l = 2 designation?
1 Answer
Explanation:
All you really need in order to answer this question is a version of the Periodic Table of Elements that shows the blocks
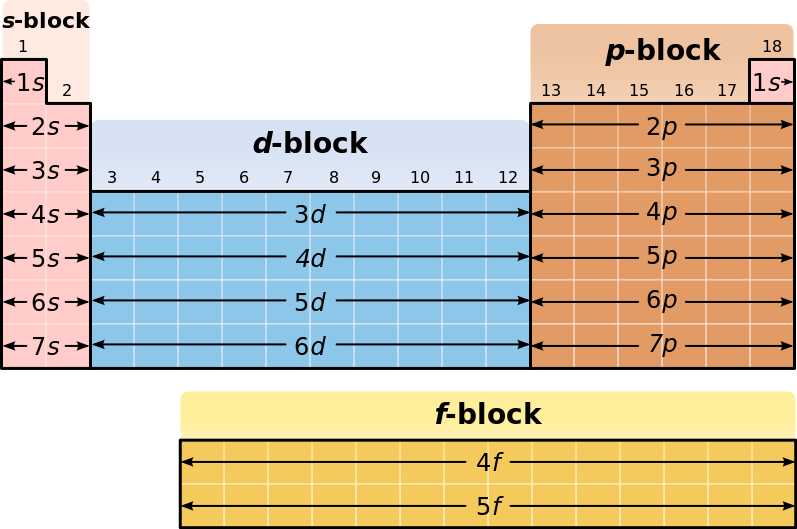
Now, the principal quantum number,
In your case,
Next, the angular momentum quantum number,
You have
#l=0 -># the s subshell#=# the s block#l=1 -># the p subshell#=# the p block#l=2 -># the d subshell#=# the d block#l=3 -># the f subshell#=# the f block
In your case,
Now, the
Therefore, a maximum of
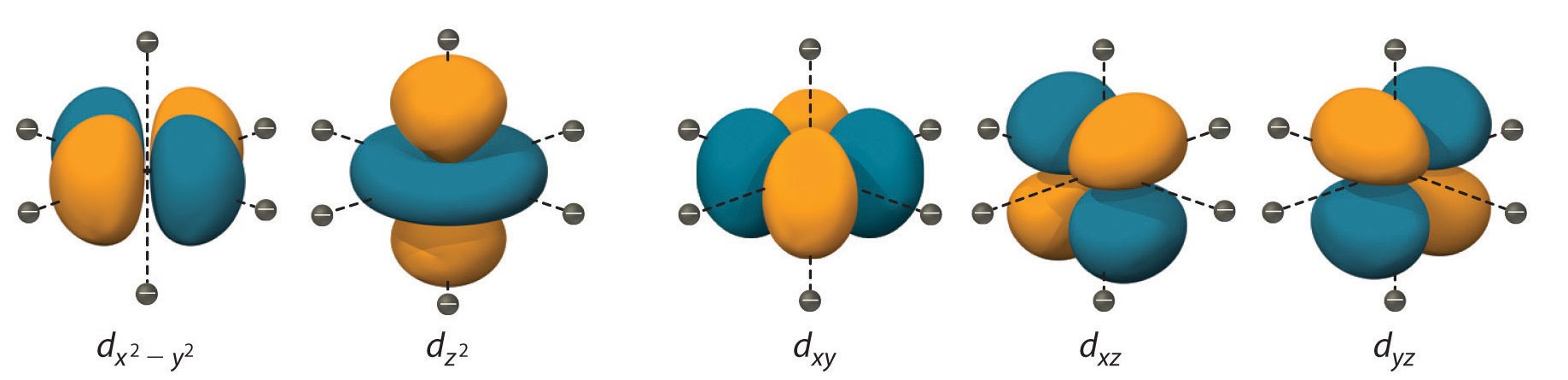
#n=5, l=2# These electrons are located on the fifth energy level, in the d subshell, i.e. in one of the
#5# d orbitals shown below
As a side note, you can find the number of orbitals that can exist in a subshell by dividing the number of groups in a block by
#"no. of orbitals in a subshell" = "no. of groups in the block"/2#
This is the case because an orbital can hold a maximum of

