What is the principal quantum number for the outermost electrons in a Ca atom in the ground state?
1 Answer
Dec 24, 2016
Explanation:
The principal quantum number,
The thing to remember about the energy level that holds an outermost electron in an atom in its ground state is that it corresponds to the period in which the element is located in the Periodic Table of Elements.
In this case, calcium,
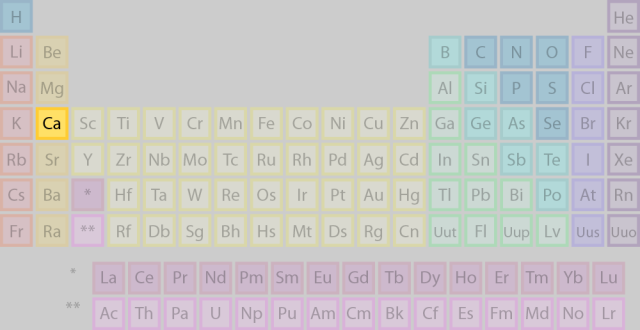
This means that for a calcium atom in its ground state, the outermost electrons are located on the fourth energy level, which, of course, implies that
#n = 4#

