A container has a volume of #12 L# and holds #14 mol# of gas. If the container is compressed such that its new volume is #7 L#, how many moles of gas must be released from the container to maintain a constant temperature and pressure?
1 Answer
Dec 27, 2016
8.17 moles of gas must be released from the container
Explanation:
Let's use Avogadro's law:
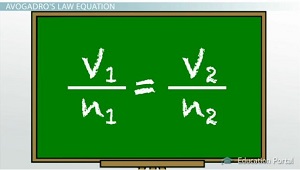
The number 1 represents the initial conditions and the number 2 represents the final conditions.
• Identify your known and unknown variables:
• Rearrange the equation to solve for the final number of moles:
• Plug in your given values to obtain the final number of moles:

