How can you find oxidation numbers of transition metals?
1 Answer
You do it in context by knowing the charges of other ligands or atoms bound to them. Always make it so the charges add up to the overall (net) charge of the compound.
Here are some examples that span general chemistry to advanced inorganic chemistry.
#"FeCl"_3#
#"Cl"^(-)# is the anion here, and there are three. Therefore:
- The oxidation state of
#"Fe"# is#+3# , and- The oxidation number is
#"III"# ,so we call this compound Iron(III) chloride.
#"Ag"_2"SO"_4#
By knowing that
#"SO"_4^(2-)# has a#2-# charge, each#"Ag"# must have:
- A
#+1# oxidation state, and- An oxidation number of
#"I"# ,to balance out the
#-2# charge. However, silver only has a#+1# oxidation state, so, we generally call this Silver sulfate.
#["Cr"("OH"_2)_6]^(3+)#
Water is clearly neutral, so chromium contributes:
- A
#+3# oxidation state.- An oxidation number of
#"III"# .The water ligand has the name "aqua", and there are six
#"H"_2"O"# 's on chromium, so we call this cation hexaaquachromium(III).Since the oxidation state is positive, we maintain the original name of the metal center.
#["Ti"("CO")_6]^(2-)#
#"CO"# , the "carbonyl" ligand, is a neutral ligand (by itself it is carbon monoxide), and when bound, contributes no charge. So, it is titanium that has:
- A
#ul(-2)# oxidation state, but still...- An oxidation number of
#"II"# .The negative sign is denoted in the name, hexacarbonyltitanate(II) (instead of hexacarbonyltitanium(II), which is
#["Ti"("CO")_6]^(2+)# and NOT the same!).
#(eta^5-"Cp")_2"Hf""Cl"_2#
This looks like this:
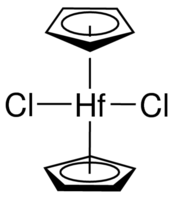
The cyclopentadienyl (
#"Cp"# ) ligands at the top and bottom are bonding via all five carbon atoms, which is what the#eta^5# is saying. We call that "pentahapto", because "penta" means five and "hapto" is how many contiguous atoms are bound to the metal at once.These
#"Cp"# ligands happen to contribute a#-1# charge, since the original complex ligand looks like this:
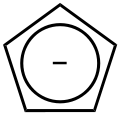
And you know that
#"Cl"^(-)# contributes a#-1# charge.So, Hafnium (
#"Hf"# ) has:
- A
#+4# oxidation state, or- An oxidation number of
#"IV"# ,to balance out the
#-4# total charge contributed by the two cyclopentadienyl and chloride ligands.Since
#"Cp"# is complex and there are two, we add the prefix "bis". As a result, we call this compound bis(pentahaptocyclopentadienyl)hafnium(IV) chloride.(the number of chlorides can be derived from the oxidation state of
#"Hf"# and the contributed charge of each#"Cp"# , so although dichloride is alright to write, it is not necessary.)

