Question #e8f2b
1 Answer
Heterolytic fission is where both electrons from a bonded pair are accepted by one atom and homolytic fission is where both atoms receive one bonded electron.
Explanation:
Homolytic fission leads to the formation of radicals. These are atoms with an unpaired electron and they are highly reactive. They take place in free radical substitution reactions.
If we add UV light to a chlorine molecule, it undergoes homolytic fission. This is the equation for the reaction:
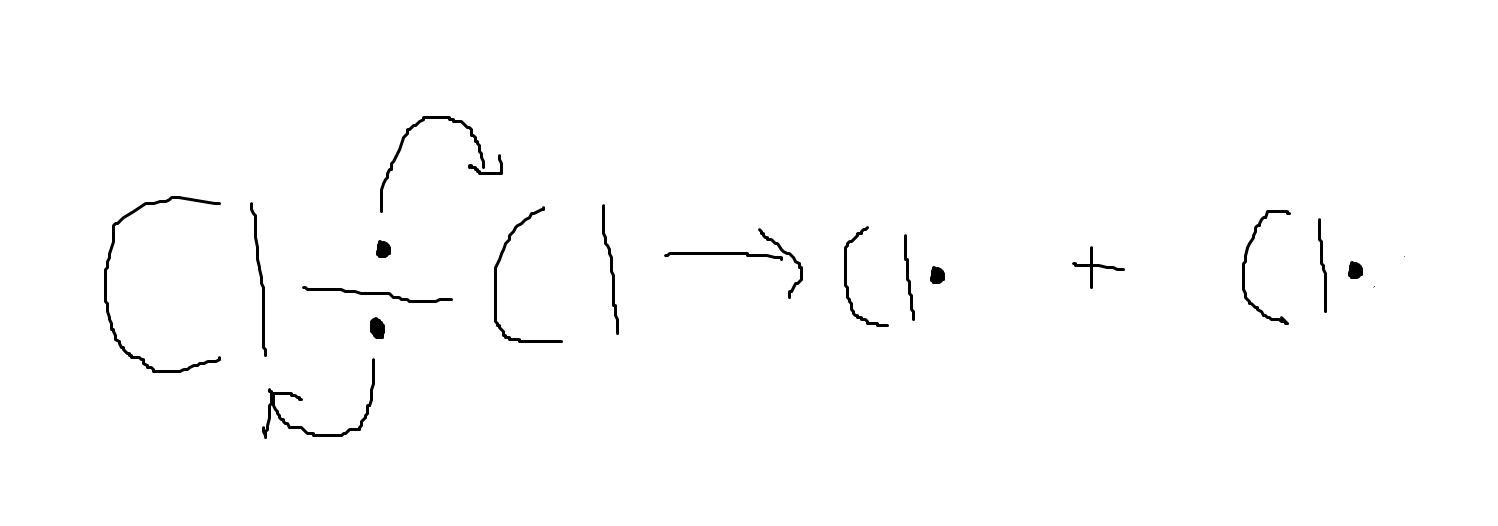
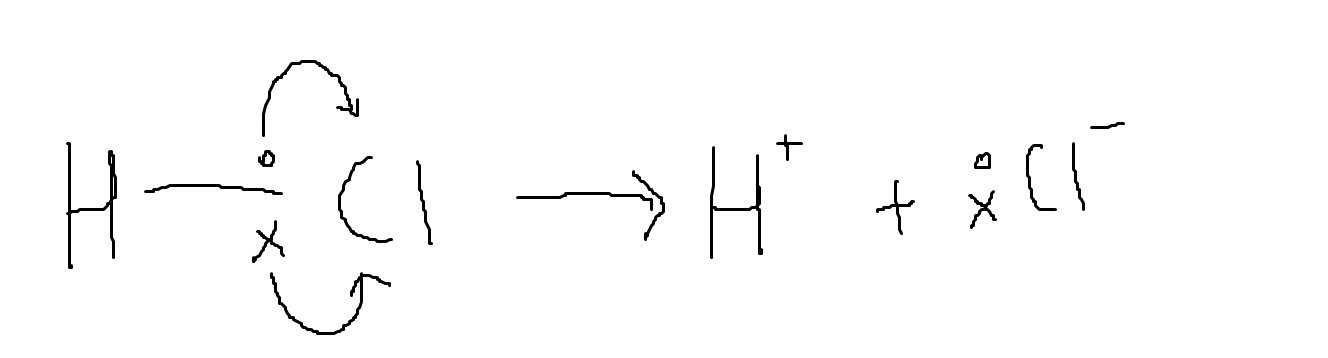
Heterolytic fission leads to the formation of two ions. One has a positive charge and the other has a negative charge. One example is the breaking of the bond in hydrochloric acid. This usually happens in the reaction between